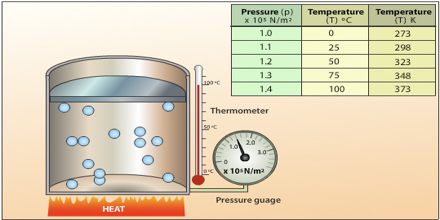
The aim of this lecture is to present on Gas Temperature, Volume and Pressure. All particles in all things are moving at all times, but some faster than others. Temperature measures the average speed of the particles in something. The Kelvin scale of temperature (K) is just like the Celsius scale (⁰C), 0 K is absolute zero; 0 K=-273 ⁰C. Absolute zero (0 K) is the coldest possible temperature. It is the temperature at which all movement stops. Here also review Boyle’s Law, Charles’s Law and Ideal Gas Law.
Gas Temperature, Volume and Pressure