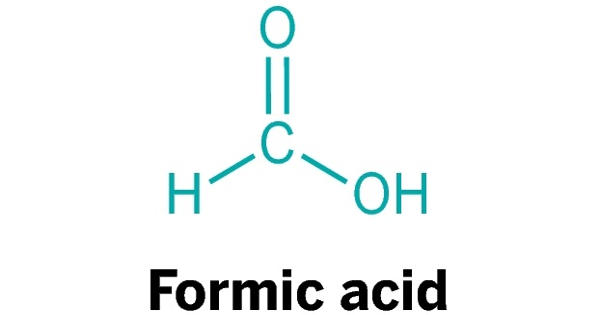
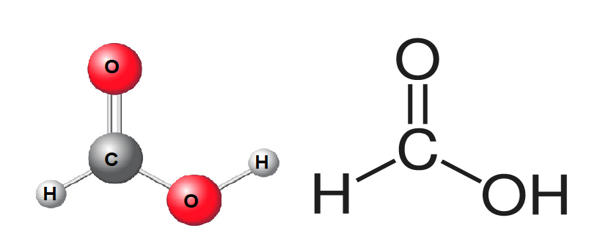
Formic Acid is a reagent comprised of the organic chemical formic acid that cleaves proteins into peptides at the C- or N-terminal side of an aspartate residue. It is systematically named methanoic acid, is the simplest carboxylic acid, and has the chemical formula HCOOH. In nature, it is found in the stings and bites of many insects of the order Hymenoptera, including bees and ants. It is an important intermediate in chemical synthesis and occurs naturally, most notably in some ants. The word “formic” comes from the Latin word for ant, Formica, referring to its early isolation by the distillation of ant bodies.
Formic acid is a colorless, fuming liquid with a pungent acrid odor with the chemical formula HCOOH.
Formic acid appears as a colorless liquid with a pungent odor. Esters, salts, and the anion derived from formic acid are called formates. Industrially, formic acid is produced from methanol. It’s dangerous at high concentrations, but at low concentrations it’s very useful.
Properties
Formic acid is a colorless liquid having a pungent, penetrating odor at room temperature, not unlike the related acetic acid. It is the simplest member of the carboxylic acid family. It’s also known as methanoic acid.
- Density: 1.22 g/cm³
- Molecular Weight/ Molar Mass: 46.03 g/mol
- Boiling Point: 100.8 °C
- Melting Point: 8.4 °C
It is miscible with water and most polar organic solvents, and is somewhat soluble in hydrocarbons. In hydrocarbons and in the vapor phase, it consists of hydrogen-bonded dimers rather than individual molecules. The molecule is composed of a carboxyl group (COOH) with a hydrogen atom attached. Owing to its tendency to hydrogen-bond, gaseous formic acid does not obey the ideal gas law. Solid formic acid, which can exist in either of two polymorphs, consists of an effectively endless network of hydrogen-bonded formic acid molecules.
Formic acid forms a low-boiling azeotrope with water (22.4%). Liquid formic acid tends to supercool. It’s flash point 156°F. It’s density 10.2 lb / gal. It’s corrosive to metals and tissue.
Natural occurrence
In nature, formic acid is found in most ants and in stingless bees of the genus Oxytrigona. In nature, it usually exists in the form of a colorless liquid. The wood ants from the genus Formica can spray formic acid on their prey or to defend the nest. The puss moth caterpillar (Cerura vinula) will spray it as well when threatened by predators. It is also found in the trichomes of stinging nettle (Urtica dioica). Formic acid is a naturally occurring component of the atmosphere primarily due to forest emissions. It occurs naturally in various sources including the venom of bee and ant stings, and is a useful organic synthetic reagent.
Uses
Humans use formic acid as a food preservative, since it’s an antibacterial substance. It’s also used to kill pests, to produce food and cosmetic additives, and to help a variety of industrial processes to occur. It principally used as a preservative and antibacterial agent in livestock feed.
- A major use of formic acid is as a preservative and antibacterial agent in livestock feed.
- In the poultry industry, it is sometimes added to feed to kill E. coli bacteria.
- Formic acid is also significantly used in the production of leather, including tanning, and in dyeing and finishing textiles, because of its acidic nature.
- Formic acid is also used in place of mineral acids for various cleaning products, such as limescale remover and toilet bowl cleaner.
Information Source: