A study on the treatment of refractory metastatic colorectal cancer has yielded results that could potentially change the standard of care for patients with this condition. The study may have found a new treatment option or a new combination of existing treatments that is more effective than the current standard of care.
Josep Tabernero, Director of the Vall d’Hebron Institute of Oncology (VHIO), presented data from the international phase III SUNLIGHT study during an Oral Abstract Session at this week’s American Society of Clinical Oncology’s (ASCO) 20th Annual Gastrointestinal Cancers Symposium, January 19-21, San Francisco, CA (U.S.).
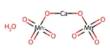
Efficacy and safety of the orally administered combination of trifluridine/tipiracil plus monoclonal antibody bevacizumab versus trifluridine/tipiracil alone in the third-line treatment of patients with refractory metastatic colorectal cancer (mCRC) who had progressed after two lines of prior therapy were the goals of this open-label controlled two-arm, phase III comparison study.
Only 11% of colon cancer patients will survive five years after diagnosis, and over 50% of them will eventually develop metastases. Patients with refractory disease continue to have a poor prognosis, with a median survival time of typically 4–8 months.
Trifluridine/tipiracil or regorafenib are now the standard of care for this patient population due to the lack of accessible therapeutic choices. Some patients may also get these medications in combination with other authorized medicines.
In addition to the magnitude of benefit observed in patients who received the combination, adding bevacizumab to trifluridine/tipiracil did not increase the risk of serious adverse or adverse events. Improved survival was observed across all subgroups independently of tumor sidedness, RAS mutational status, and receipt of prior bevacizumab, indicating that the combination regimen is an option for all clinically relevant subgroups.
Elena Élez
“There is an urgent, unmet clinical need to identify new and more effective treatment strategies to improve the survival and quality of life of our patients. Representing an important step in this direction, the SUNLIGHT phase III study was designed to evaluate the true clinical value of combining trifluridine/tipiracil with bevacizumab,” says Josep Tabernero, first author of this present study and Head of the Vall d’Hebron University Hospital’s (HUVH) Medical Oncology Department, Vall d’Hebron Barcelona Hospital Campus.
SUNLIGHT enrolled 492 patients across 103 study locations who had received a maximum of two prior chemotherapy regimens for the treatment of advanced colorectal cancer and had demonstrated progressive disease or intolerance to their last regimen. These patients were randomized (1:1) to receive trifluridine/tipiracil in combination with bevacizumab, or trifluridine/tipiracil monotherapy.
The overall survival endpoint served as the primary outcome measure for this phase III clinical study, with overall response rate, disease control rate, treatment-emergent adverse events, and quality of life serving as supplementary outcome measures.
Potentially practice-changing data
The SUNLIGHT study investigators, including Elena Élez, a Senior Researcher of VHIO’s Gastrointestinal and Endocrine Tumors Group, reported an improved median survival of 3.3 months with trifluridine/tipiracil plus bevacizumab, from 7.5 months with trifluridine/tipiracil monotherapy to 10.8 months with the combination regimen.
“An improved survival rate of over three months in metastatic colorectal cancer is considered as statistically as well as clinically relevant. These present data could therefore promise a new standard of care for patients with refractory disease who have progressed after two prior lines of therapy,” observes Tabernero.
Trifluridine/tipiracil alone resulted in a progression-free survival of 2.4 months compared to 5.6 months when coupled with bevacizumab. Time to global health status degradation was 4.7 and 8.5 months, respectively.
Quality of life was graded according to the ECOG Performance Status Scale. In individuals taking trifluridine/tipiracil alone, the median time to deteriorating to a grade 2 or above was 6.3 months as opposed to 9.3 months with the combination.
“In addition to the magnitude of benefit observed in patients who received the combination, adding bevacizumab to trifluridine/tipiracil did not increase the risk of serious adverse or adverse events. Improved survival was observed across all subgroups independently of tumor sidedness, RAS mutational status, and receipt of prior bevacizumab, indicating that the combination regimen is an option for all clinically relevant subgroups,” adds Elena Élez, a Senior Consultant of HUVH’s Medical Oncology Department.
By demonstrating improved efficacy over an active regimen in these patients, with a greater benefit than that seen in other trials, and in a population that included patients with poor prognostic factors like RAS mutations, SUNLIGHT is the first phase III study to validate a new third-line treatment strategy.
“Our data point to trifluridine/tipiracil plus bevacizumab as a new standard of care. This combination therapy could therefore open a much needed, more effective treatment avenue for patients with refractory metastatic colorectal cancer who have progressed after two lines of therapy,” concludes Josep Tabernero.