Feldspars (LiAlSi3O8 – NaAlSi3O8 – CaAl2Si2O8) are a group of rock-forming tectosilicate minerals that make up about 41% of the Earth’s continental crust by weight. The feldspars are a group of minerals that have similar characteristics due to a similar structure. It is a group of minerals, principally aluminosilicates of potassium, sodium, and calcium, characterized by two cleavages at nearly right angles: one of the most important constituents of igneous rocks.
Feldspars crystallize from magma as veins in both intrusive and extrusive igneous rocks and are also present in many types of metamorphic rock. Rock formed almost entirely of calcic plagioclase feldspar is known as anorthosite. Feldspars are also found in many types of sedimentary rocks.
General Information
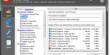
- Category: Tectosilicate
- Formula: (LiAlSi3O8 – NaAlSi3O8 – CaAl2Si2O8)
- Crystal system: Triclinic or monoclinic

Feldspars
Properties
They are slightly hard at around 6 and have an average density of 2.55 to 2.76. They have a rather dull to the rarely vitreous luster. Crystals tend to be blocky. Some feldspars may be triboluminescent. They have two directions of cleavage at nearly right angles. Feldspars also tend to crystallize in igneous environments, but are also present in many metamorphic rocks.
- Color: pink, white, gray, brown
- Cleavage: two or three
- Fracture: along cleavage planes
- Mohs scale hardness: 6.0–6.5
- Luster: Vitreous
- Streak: white
- Diaphaneity: opaque
- Specific gravity: 2.55–2.76
- Density: 2.56
Feldspars play an important role as fluxing agents in ceramics and glass applications, and also are used as functional fillers in the paint, plastic, rubber and adhesive industries.
Feldspar is a common raw material used in glassmaking, ceramics, and to some extent as a filler and extender in paint, plastics, and rubber. In glassmaking, alumina from feldspar improves product hardness, durability, and resistance to chemical corrosion. In ceramics, the alkalis in feldspar (calcium oxide, potassium oxide, and sodium oxide) act as a flux, lowering the melting temperature of a mixture.
Information Source: