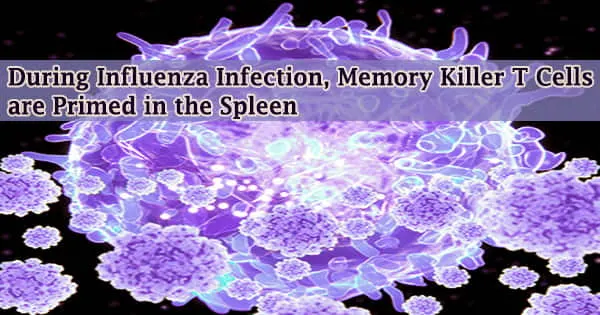
CD8+ T cells, also known as “killer” T cells, are the immune system’s assassins. They seek out and destroy other cells that are infected with virus or malignant once they have been primed.
Dendritic cells serve as immune system sentinels during priming. In the case of an influenza infection in the lungs, lung-migratory dendritic cells acquire a fragment of viral antigen and subsequently migrate out of the lung to a location where naive T cells exist, where the antigen is presented to CD8+ T cells. This prepares T cells to attack certain cells.
Long assumed to be limited to a single anatomical site, the lung-draining, mediastinal lymph nodes that lie between the lungs and the spine, priming in influenza was thought to be limited to a single anatomical region. In a publication published in the journal Science Immunology, this lymph node-centric paradigm has been challenged.
The spleen has been discovered to be an undiscovered extra site for priming CD8+ T lymphocytes, according to researchers lead by André Ballesteros-Tato, Ph.D., associate professor in the University of Alabama at Birmingham Department of Medicine Division of Clinical Immunology and Rheumatology.
This is both shocking and crucial. It’s surprising because the lungs and the spleen have no lymphatic vessels in common. Important, says Ballesteros-Tato, since CD8+ T cells primed there are transcriptionally distinct from lymph node T cells and are destined for a different fate. CD8+ T cells primed in lymph nodes are on their way to become T effector cells that will fight infection in the lungs.
We demonstrate that CD8+ T cells, responding to the same antigen in different anatomical locations, gives rise to cells with distinct functional capabilities; thereby providing a new ‘anatomical model’ for how T cell diversity is generated after infection.
André Ballesteros-Tato
Those that are stimulated in the spleen, on the other hand, produce precursors that are better able to develop into long-lived, stem-like memory T cells. Such memory cells are capable of responding quickly in the event of a future flu virus infection, giving long-term protective immunity.
“We demonstrate that CD8+ T cells, responding to the same antigen in different anatomical locations, gives rise to cells with distinct functional capabilities,” Ballesteros-Tato said, “thereby providing a new ‘anatomical model’ for how T cell diversity is generated after infection.
“Our results demonstrate a dendritic cell-trafficking pathway that connects the lung with the blood circulation and identify the spleen as a primary site for the priming of long-lived memory T cell precursors,” he said. “These data will be critical for the development of more efficient vaccination and therapeutic strategies to respiratory challenges.”
Using a mouse/influenza virus infection model, the UAB researchers discovered that a fraction of the migratory lung dendritic cells that exit the lymph node, enter the blood stream, and return to the spleen were responsible for priming in the spleen.
Apart from transcriptional differences between spleen-primed CD8+ T cells and CD8+ T cells primed in mediastinal lymph nodes, fate-mapping tests revealed that spleen-primed CD8+ T cells were long-lived and contributed significantly to the pool of long-lived memory cells.
Ballesteros-Tato and colleagues also discovered that 45 days after infection, when spleen-primed CD8+ T cells and lymph node-primed CD8+ T cells were phenotypically indistinguishable, the spleen-primed T cells had a superior capacity to respond to an influenza virus rechallenge infection and expand into an infection-fighting T effector cell population.
Researchers discovered that when dendritic cells could not migrate out of the lung or lymph node egress was blocked, dendritic cells expressing lung-derived antigens failed to collect in the spleen, inhibiting splenic T cell priming.
But how do migratory dendritic cells in the lungs move from lymph nodes to spleen?
“Neither the mediastinal lymph nodes nor the lungs are directly connected to the spleen by the lymphatic vasculature,” Ballesteros-Tato said. “As a result, the thoracic duct, which conducts efferent lymph back into the blood circulation, is the only way for migratory dendritic cells to reach the spleen from the mediastinal lymph nodes.”
Ballesteros- The majority of what we know about stem-like CD8+ T cells comes from research in tumor models or persistent systemic viral infections, according to Tato.
“To this day,” he said, “no studies have been performed to address the question of how these cells are generated in the context of respiratory viral infections.”