One of the first successful human studies of an anti-amyloid therapy, a sizable trial of an experimental Alzheimer’s disease medicine has showed promise in reducing cognitive deterioration over an 18-month period.
The results, which Biogen and Eisai announced on Tuesday, indicated a 27 percent slower progression of Alzheimer’s disease with the medicine compared to placebo. The results couldn’t have arrived at a better moment after a string of unsuccessful anti-amyloid medication trials.
The outcomes might finally add some hard data to the amyloid theory, according to Eisai.
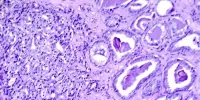
The abnormal buildup of A in the brain is one of the primary causes of Alzheimer’s disease, according to the amyloid hypothesis, which is supported by the lecanemab Clarity AD research results, according to Haruo Naito, chief executive officer at Eisai.
“Eisai believes that these findings may open up new possibilities for the identification and management of Alzheimer’s disease and will further stimulate research into novel therapeutic approaches.”
Lecanemab is the name of the medication; it is an anti-amyloid beta (A) protofibril antibody therapy that works to reduce plaques of misfolded protein in the brain that are thought to be the cause of the cognitive decline seen in Alzheimer’s and disorders that resemble it.
The research aim was a significant slowing of disease progression, and the trial enrolled nearly 1,800 people with amyloid plaques in the brain.
The trial demonstrated significant improvements as early as six months in, with a reduction in clinical decline of 27% after 18 months compared to placebo.
When it comes to side effects, lecanemab considerably outperformed placebo groups, with 12.5 percent of the lecanemab group reporting amyloid-related imaging abnormalities-edema/effusion (ARIA-E), an antibody-related accumulation of fluid in the brain. While none of those in the placebo group showed symptoms, 2.8% of those in the lecanemab group did.
Following the encouraging Phase 3 study results, the researchers will now strive for approval in the US and Japanese markets, but the ramifications for Alzheimer’s research are undoubtedly significant.
Importantly, the research demonstrates that removing amyloid beta aggregates from the brain can reduce the progression of the disease in those who are still in the early stages of the condition. According to Michel Vounatsos, CEO of Biogen, “We wish to thank the many patients who took part in this historic worldwide study and want to congratulate the clinical investigators who worked tirelessly to boost the inclusion of historically underrepresented communities.”