A new class of motorized molecules that kill specific bacteria appears to have the potential to reduce the threat of antibiotic resistance to human health. A team led by Rice University scientists developed light-activated hemithioindigo (HTI) molecules that kill Gram-positive bacteria and the biofilm they form. The molecules accomplish this by increasing the local production of reactive oxygen species (ROS), which attack and destroy drug-resistant cells chemically.
The new molecules differ from and complement others developed at Rice that are also light activated but drill into cell membranes to kill them. The HTI-based molecules, like the drills based on Nobel Prize-winning work by Bernard Feringa, are activated by visible light rather than harmful ultraviolet radiation.
Both are the work of Rice University chemist James Tour and his colleagues. Ana Santos, a postdoctoral global fellow at the Health Research Institute of the Balearic Islands in Palma, Spain, and Alexis van Venrooy, now a senior scientist at Genesis Therapeutics in San Diego, are the study’s co-lead authors.
A significant advantage of these molecules is that they have a narrow spectrum of activity and kill a specific group of bacteria, Gram-positive bacteria. As a result, they are less likely to cause the side effects seen with broad-spectrum antibiotics, which kill both ‘bad’ and ‘good’ bacteria indiscriminately, and they are also less likely to lead to resistance because only one group of bacteria is affected.
Ana Santos
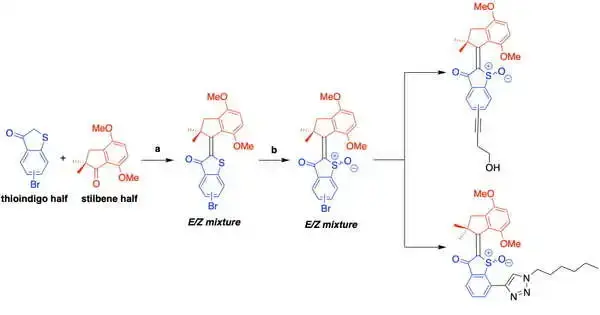
The HTI-based molecular machines are made up of two halves: a thioindigo unit connected to a carbocycle via a central carbon double bond and a carbocycle. Depending on the molecular design, when triggered by visible light, the molecule undergoes a conformational change that results in either a drill-like 360-degree motion or a shift between two conformations, similar to a “on/off” switch.
In the process, activated HTIs react with the cell and molecular oxygen, transferring electrons to produce ROS that batter the target cells.
“These are not killing cells by mechanically ripping open the membranes like the earlier ones do,” Tour said. “They induce enough disruption that reactive oxygen species and free radicals are generated and end up killing the cells. So it’s not the fast necrotic death that we saw before,” he said. “It’s a little bit slower, but it’s extremely efficient.”

“A significant advantage of these molecules is that they have a narrow spectrum of activity and kill a specific group of bacteria, Gram-positive bacteria,” Santos explained. “As a result, they are less likely to cause the side effects seen with broad-spectrum antibiotics, which kill both ‘bad’ and ‘good’ bacteria indiscriminately, and they are also less likely to lead to resistance because only one group of bacteria is affected.”
Gram-positive bacteria lack an outer membrane (though they have a thick peptidoglycan layer), making them more vulnerable to ROS that oxidize and degrade their cell walls.
“A significant advantage of these molecules is that they have a narrow spectrum of activity and kill a specific group of bacteria, Gram-positive bacteria,” Santos explained. “As a result, they are less likely to cause the side effects seen with broad-spectrum antibiotics, which kill both ‘bad’ and ‘good’ bacteria indiscriminately, and they are also less likely to lead to resistance because only one group of bacteria is affected.”
Gram-positive bacteria lack an outer membrane (though they have a thick peptidoglycan layer), making them more vulnerable to ROS that oxidize and degrade their cell walls.
The study showed that HTIs also killed antibiotic-tolerant persister cells of different Gram-positive strains in as little as 25 minutes, faster than conventional antibiotics. In every case, repeated exposure to HTIs did not increase the bacteria’s resistance to treatment.
According to Santos, the treatment does not harm mammalian cells because it is based on ROS rather than mechanical action. “This paves the way for a new antimicrobial therapy that can safely target Gram-positive pathogens associated with skin infections such as burn wounds,” she said.
“The findings also contribute to our understanding of molecular machines in general by demonstrating that not all of them act by the same mechanisms and that differences in the chemical core of the molecule can result in very different biological actions.”