Dichlorine monoxide is a molecular formula Cl2O inorganic compound. It is a reddish gas which is a dark brown liquid, at temperatures below freezing. It was first synthesized in 1834 by Antoine Jérôme Balard who also determined his composition along with Gay-Lussac. In older literature, it is sometimes referred to as chlorine monoxide, which can be a source of confusion because that term now refers to the neutral species ClO.
Dichlorine monoxide (Cl2O) is one of the makers of oxychlorine acids, called “chlorine oxide”. It occurs at room temperature as a brownish-yellow gas and is both soluble in water and organic solvents. Chemically, it is a member of the compounds chlorine oxide class, as well as being the hypochloric acid anhydride. It is a good chlorinating and oxidizing agent.
Dichlorine monoxide (Cl2O) is a brownish, yellow gas at room temperature that can combust when heated or exposed to sparks at high concentrations. It is best prepared by treating fresh yellow mercuric oxide with Cl2 gas:
2Cl2+2HgO→HgCl2·HgO+Cl2O
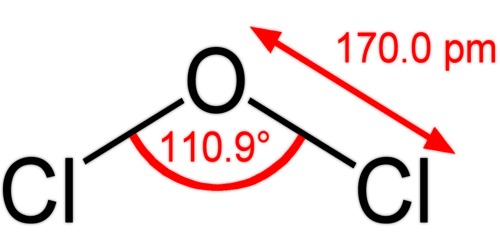
The composition of dichlorine monoxide is identical to that of water and hypochloric acid, and due to the lone pairs on the oxygen, the molecule adopts a bent molecular geometry; resulting in C2V molecular symmetry. The angle of the bond is slightly larger than natural, presumably due to steric repulsion between the voluminous chlorine atoms. It can also be prepared by reaction of Cl2 gas with moist sodium carbonate, Na2CO3.
Dichlorine monoxide is highly soluble in liquids. That is the hypochloric acid anhydride. It crystallizes in the solid-state in the tetrahedral space group I41/amd, rendering it isostructural to water, ice VIII, in the high-pressure form. Most of the industrially produced Cl2O is used for producing hypochlorites. Dichlorine monoxide is highly soluble in water, where it is present in combination with HOCl. Dichlorine monoxide has been proposed because of the active species within the reactions of HOCl with olefins and aromatic compounds, likewise as within the chlorination of beverage.
Often, dichlorine monoxide is the unstable form of calcium hypochlorite in decomposition. It is an effective chlorinating agent; it can be used in deactivated aromatic substrates for either the side-chain or ring chlorination. Dichlorine monoxide is explosive although this activity has been missing in modern science. Room temperature mixtures with oxygen couldn’t be detonated by an electrical spark until they contained a minimum of 23.5% Cl2O; which is an exceedingly high minimum explosive limit.
Information Sources: