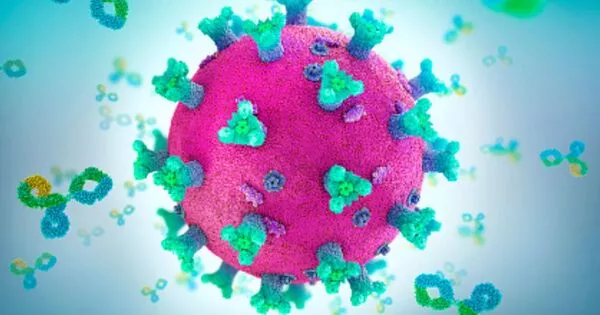
Understanding the structures of lethal viruses can provide useful insights and open up new avenues for vaccine development. A virus’s structure, including its proteins and other components, is critical to its ability to infect cells and cause disease. Scientists can identify potential targets for vaccines to elicit an immune response and neutralize the virus by studying these structures.
A Scripps Research team discovered new antibodies and vaccine targets by comparing the structures of protein complexes from different lineages of the dangerous Lassa virus.
Hundreds of thousands of people in West Africa are infected with the Lassa virus each year, which can cause Lassa fever and lead to severe illness, long-term side effects, or death. There are no widely accepted treatments or vaccines for the disease at the moment. Scripps Research scientists have now determined the structure of the critical protein complex that allows Lassa virus to infect human cells. The study, which was published online in Cell Reports, also discovered new antibodies that bind to these proteins and neutralize the virus, paving the way for more effective Lassa virus vaccines and treatments.
“This work is a big step forward in our ability to isolate new antibodies to relevant sites of vulnerability on the virus, and it provides a basis to conduct rational vaccine design to broadly protect people against many lineages of the Lassa virus,” says senior author Andrew Ward, PhD, professor of Integrative Structural and Computational Biology at Scripps Research. “These new reagents described in the paper are already being put to good use and yielding exciting new results.”
This work is a big step forward in our ability to isolate new antibodies to relevant sites of vulnerability on the virus, and it provides a basis to conduct rational vaccine design to broadly protect people against many lineages of the Lassa virus.
Andrew Ward
Lassa virus, like many other viruses, exists in several lineages, each with slight variations in its genes. Because of this variety, it has been difficult to identify antibodies that recognize all variants of the Lassa virus. Scientists have also had difficulty isolating Lassa glycoproteins, which are spike-like proteins that surround the virus and are the target of most antibodies. These glycoproteins are found in the infectious virus in three-protein complexes known as trimers. However, scientists were only able to isolate glycoproteins in the lab as single proteins, not in their trimer complexes, for decades.
Ward and colleagues discovered how to use nanoparticles to hold glycoprotein trimers together in 2022. They used that technique in the new study to isolate and structurally characterize trimers of the glycoproteins from four different Lassa virus lineages. Surprisingly, the glycoprotein structures from the various lineages were strikingly similar.
“We were hoping to see more obvious differences that would explain why antibodies didn’t recognize all the lineages,” says Hailee Perrett, the study’s first author and a Scripps Research graduate student. “Instead, we found a very high level of conservation across the peptide and sugar components of the protein.”
Ward, Perrett, and their colleagues then isolated antibodies against the glycoprotein trimers using blood samples from patients who had recovered from Lassa virus. They discovered new antibodies and characterized previously discovered antibodies that recognize different lineages of the Lassa virus glycoprotein, which could aid in the development of a treatment or preventive vaccine against the virus.
Future experiments are already being planned to identify more antibodies against the Lassa virus glycoproteins, as well as further analyzing the protein structures to identify places on the glycoproteins that are ideal for drug targeting.
“Our goals were not only to try and define some of the structural details of these different Lassa viruses, but also to provide foundational protocols and resources for the field,” Perrett explains. “We hope that our approaches and preliminary findings contribute to the advancement of science in this field.”