While many people wonder how to prevent prostate cancer, there is no single way to avoid the disease. Staying healthy as you age, or working to reverse existing health problems, can reduce your risk. However, prostate cancer, like all cancers, has certain risk factors that cannot be avoided.
In a recent trial, nine patients whose tumors were resistant to androgen-blocking therapy were given that therapy along with a CD105 inhibitor called carotuximab. Based on radiographic imaging, forty percent of those patients had progression-free survival.
Cedars-Sinai Cancer Center researchers have identified an investigational therapeutic approach that may be effective against treatment-resistant prostate cancer. The findings of their Phase II clinical trial, which were published in the peer-reviewed journal Molecular Therapy, have prompted a larger, multicenter trial, which will begin soon.
Prostate cancer, which affects a small gland just below the bladder, is the second leading cause of cancer death in men. Many prostate tumors are benign and require no or little treatment. Surgery or radiation therapy are used to treat aggressive tumors at first.
We discovered that this therapy may be able to resensitize select patients to androgen suppression, particularly in early cancers. This could allow patients to avoid or postpone more harmful interventions like cytotoxic chemotherapy.
Edwin Posadas
In about one-third of patients, cancer comes back after initial treatment, said Neil Bhowmick, Ph.D., research scientist at Cedars-Sinai Cancer, professor of Medicine and Biomedical Sciences, and senior author of the study. Those patients are usually treated with medications that suppress the actions of testosterone and other androgens – male hormones that help prostate tumors grow.
“Patients do really well until the tumor figures a way around the androgen-suppressing therapy,” Bhowmick said. “One way that it can do this is to cause cells to make only part of the protein that the drug binds to, rendering the drug useless. The partial proteins are called splice variants.”
Through research with human cells and laboratory mice, study first author Bethany Smith, Ph.D., a project scientist in the Bhowmick Lab, figured out that the cancer cells were signaling to the surrounding supportive cells through a protein called CD105 to make these slice variant proteins. Investigators then conducted a trial in human patients to test a drug that they hoped would keep those partial proteins from forming by inhibiting CD105.
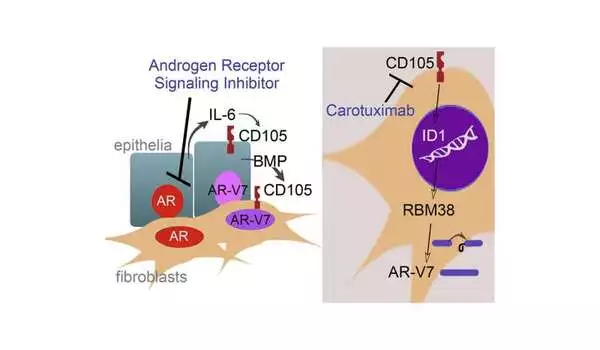
In the trial, nine patients whose tumors were resistant to androgen-blocking therapy continued that therapy but were also given a CD105 inhibitor called carotuximab. Forty percent of those patients experienced progression-free survival, based on radiographic imaging.
“Every single patient in our trial was completely resistant to at least one androgen suppressor, and the normal course of action would be to simply try another one or chemotherapy, which research has shown generally does not stop tumor growth for more than about three months,” Bhowmick explained. “Carotuximab inhibited cancer’s workaround and rendered the tumor susceptible to androgen-suppressive therapy.”
Importantly, carotuximab appears to prevent androgen receptor splice variants in supporting cells surrounding tumors, further sensitizing the tumor to the androgen suppressor, according to Bhowmick.
“We discovered that this therapy may be able to resensitize select patients to androgen suppression, particularly in early cancers. This could allow patients to avoid or postpone more harmful interventions like cytotoxic chemotherapy” According to Edwin Posadas, MD, co-director of the Experimental Therapeutics Program, medical director of the Urologic Oncology Program/Center for Uro-Oncology Research Excellence (CURE), associate professor of Medicine at Cedars-Sinai, and study co-author. “We also hope to find ways to predict which patients will benefit the most from this approach by testing blood and tissue samples with next-generation technologies housed at Cedars-Sinai Cancer Center.”