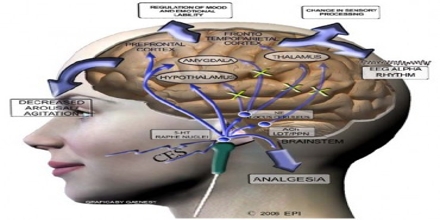
Cranial Electrotherapy Stimulation technology is classified by the Food and Drug Administration like a Class III medical device and must be dispensed by or within the order of an authorized healthcare practitioners. Cranial electrotherapy stimulation (CES) is a sort of non-invasive brain arousal that applies a compact, pulsed electric current across the head to treat anxiety, depression, sleep loss and chronic soreness.
Cranial Electrotherapy Stimulation