Cobalt-free batteries are a promising advancement in the pursuit of cleaner and more environmentally friendly power storage technologies. Because of its extraction procedure and the fact that a major amount of global cobalt production comes from regions with poor labor and environmental standards, cobalt, a vital component in many lithium-ion batteries, has prompted environmental and ethical concerns.
Rechargeable batteries with high capacity and dependability are essential components of many electronics and even means of transportation. They are critical to the transition to a greener world. They are made from a wide range of elements, including cobalt, the manufacture of which contributes to some environmental, economic, and social difficulties. For the first time, a team led by experts from the University of Tokyo provides a potential alternative to cobalt that can exceed current battery chemistry in several aspects. It can also withstand many recharge cycles, and the basic theory can be extended to other issues.
You are most likely viewing this post on a laptop or smartphone, and if not, you own at least one of these devices. A lithium-ion battery (LIB) is found inside both of these devices, as well as many more. LIBs have been the standard method of powering portable or mobile electronic devices and machinery for decades. As the world moves away from fossil fuels, they are considered as a critical step for use in electric automobiles and house batteries for individuals who have solar panels. However, much as batteries have a positive and a negative end, LIBs have negative points offset by positive ones.
We are delighted with the results so far, but getting here was not without its challenges. It was a struggle trying to suppress various undesirable reactions that were taking place in early versions of our new battery chemistries which could have drastically reduced the longevity of the batteries.
Professor Atsuo Yamada
For one point, while being some of the most power-dense portable power sources today, many users wish LIBs had a higher energy density in order to last longer or power more demanding devices. They can also withstand a significant number of recharge cycles, but they degrade over time; it would be preferable for everyone if batteries could withstand more recharge cycles and keep their capabilities for a longer period of time. The most concerning issue with modern LIBs, however, is one of the materials employed in their construction.
Cobalt is commonly utilized in the electrodes, which are an important component of LIBs. All batteries function in the same way: When connected to an external circuit, two electrodes, one positive and one negative, facilitate the movement of lithium ions between them in what is known as the electrolyte. Cobalt, on the other hand, is a rare element, so uncommon that there is now just one major source of it: a network of mines in the Democratic Republic of the Congo. Many questions have been raised throughout the years regarding the environmental repercussions of these mines, as well as the working conditions, including the use of child labor. Because of the region’s political and economic instability, the source of cobalt is also an issue.
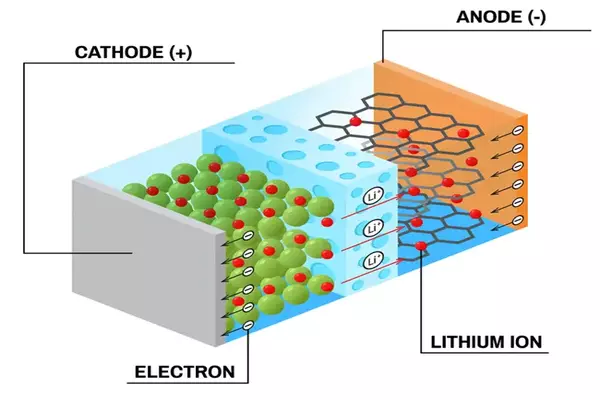
“There are many reasons why we want to transition away from using cobalt in order to improve lithium-ion batteries,” stated Professor Atsuo Yamada of the Department of Chemical System Engineering. “We face a technical challenge, but the impact could be environmental, economic, social, and technological. We are thrilled to present a new cobalt alternative that employs a novel combination of metals in the electrodes, including lithium, nickel, manganese, silicon, and oxygen – all of which are significantly more common and less difficult to create and deal with.”
Yamada and his team’s novel electrodes and electrolyte are not only cobalt-free, but they also improve on current battery chemistry in several aspects. The new LIBs have a 60% higher energy density, which could imply a longer life, and can deliver 4.4 volts, as opposed to 3.2-3.7 volts in typical LIBs. However, one of the most astonishing technological successes was the enhancement of recharge properties. The new chemistry allowed test batteries to fully charge and discharge over 1,000 cycles (simulating three years of full use and charging) while losing only roughly 20% of their storage capacity.
“We are delighted with the results so far, but getting here was not without its challenges. It was a struggle trying to suppress various undesirable reactions that were taking place in early versions of our new battery chemistries which could have drastically reduced the longevity of the batteries,” said Yamada. “And we still have some way to go, as there are lingering minor reactions to mitigate in order to improve the safety and longevity even further. At present, we are confident that this research will lead to improved batteries for many applications, but some, where extreme durability and lifespan are required, might not be satisfied just yet.”
Although Yamada and his colleagues were investigating LIB applications, the concepts underlying their recent development can be applied to other electrochemical processes and devices, such as other types of batteries, water splitting (to produce hydrogen and oxygen), ore smelting, electro-coating, and more.