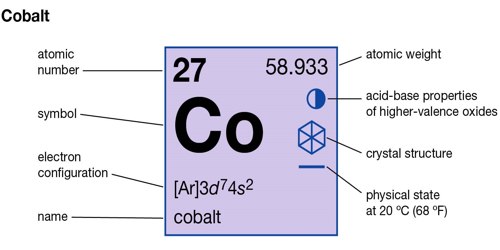
Cobalt (Co) is a chemical element, and its atomic number 27, ferromagnetic metal of Group 9 (VIIIb) of the periodic table, used especially for heat-resistant and magnetic alloys. Like nickel, cobalt is found in the Earth’s crust only in chemically combined form, save for small deposits found in alloys of natural meteoric iron. The free element, produced by reductive smelting, is a hard, lustrous, silver-gray metal.
In pure form, cobalt is silvery-blue and brittle. It is similar to iron and nickel, according to the Environmental Protection Agency, and like iron can be made magnetic. As a result, some high-powered magnets are made from alloys of cobalt and aluminum or nickel, according to the Royal Society of Chemistry. A manmade isotope, Cobalt-60, is commonly used in cancer treatments; the gamma radiation released by this radioactive isotope can target tumors, according to the American Brain Tumor Association, particularly brain tumors that need precision treatment.
The metal was isolated (c. 1735) by Swedish chemist Georg Brandt, though cobalt compounds had been used for centuries to impart a blue color to glazes and ceramics. Cobalt has been detected in Egyptian statuettes and Persian necklace beads of the 3rd millennium BCE, in glass found in the Pompeii ruins, and in China as early as the Tang dynasty (618–907 CE) and later in the blue porcelain of the Ming dynasty (1368–1644). The name kobold was first applied (16th century) to ores thought to contain copper but eventually found to be poisonous arsenic-bearing cobalt ores. Brandt finally determined (1742) that the blue color of those ores was due to the presence of cobalt.
Today, some cobalt is produced specifically from one of a number of metallic-lustered ores, such as cobaltite (CoAsS). The element is, however, more usually produced as a by-product of copper and nickel mining. The copper belt in the Democratic Republic of the Congo (DRC) and Zambia yields most of the global cobalt production. World production in 2016 was 116,000 tonnes (according to Natural Resources Canada), and the DRC alone accounted for more than 50%.
Cobalt is primarily used in lithium-ion batteries, and in the manufacture of magnetic, wear-resistant and high-strength alloys. The compounds cobalt silicate and cobalt(II) aluminate (CoAl2O4, cobalt blue) give a distinctive deep blue color to glass, ceramics, inks, paints, and varnishes. Cobalt occurs naturally as only one stable isotope, cobalt-59. Cobalt-60 is a commercially important radioisotope, used as a radioactive tracer and for the production of high-energy gamma rays.
Cobalt is the active center of a group of coenzymes called cobalamins. Vitamin B12, the best-known example of the type, is an essential vitamin for all animals. Cobalt in inorganic form is also a micronutrient for bacteria, algae, and fungi.
Fact –
According to the Jefferson National Linear Accelerator Laboratory, the properties of cobalt are:
- Atomic number (number of protons in the nucleus): 27
- Atomic symbol (on the Periodic Table of Elements): Co
- Atomic weight (average mass of the atom): 58.933195
- Density: 8.86 grams per cubic centimeter
- Phase at room temperature: Solid
- Melting point: 2,723 degrees Fahrenheit (1,495 degrees Celsius)
- Boiling point: 5,301 F (2,927 C)
- Number of isotopes (atoms of the same element with a different number of neutrons): 8; 1 stable
- Most common isotopes: Co-59 (100 percent natural abundance)
Occurrence and Properties of Cobalt –
Cobalt, though widely dispersed, makes up only 0.001 percent of Earth’s crust. It is found in small quantities in terrestrial and meteoritic native nickel-iron, in the Sun and stellar atmospheres, and in combination with other elements in natural waters, in ferromanganese crusts deep in the oceans, in soils, in plants and animals, and in minerals such as cobaltite, linnaeite, skutterudite, smaltite, heterogenite, and erythrite. In animals, cobalt is a trace element essential in the nutrition of ruminants (cattle, sheep) and in the maturation of human red blood cells in the form of vitamin B12, the only vitamin known to contain such a heavy element.
Cobalt in compound form occurs in copper and nickel minerals. It is the major metallic component that combines with sulfur and arsenic in the sulfidic cobaltite (CoAsS), safflorite (CoAs2), glaucodot ((Co,Fe)AsS), and skutterudite (CoAs3) minerals. The mineral cattierite is similar to pyrite and occurs together with vaesite in the copper deposits of Katanga Province. When it reaches the atmosphere, weathering occurs; the sulfide minerals oxidize and form pink erythrite (“cobalt glance”: Co3(AsO4)2·8H2O) and spherocobaltite (CoCO3).
Cobalt is also a constituent of tobacco smoke. The tobacco plant readily absorbs and accumulates heavy metals like cobalt from the surrounding soil in its leaves. These are subsequently inhaled during tobacco smoking. By the second decade of the 21st century, the Democratic Republic of the Congo (DRC), China, Canada, and Russia were the world’s leading producers of mined cobalt. The largest producer of refined cobalt, however, was China, which imported vast additional amounts of cobalt mineral resources from the DRC.
Polished cobalt is silver-white with a faint bluish tinge. Two allotropes are known: the hexagonal close-packed structure, stable below 417 °C (783 °F), and the face-centred cubic, stable at high temperatures. It is ferromagnetic up to 1,121 °C (2,050 °F, the highest known Curie point of any metal or alloy) and may find application where magnetic properties are needed at elevated temperatures.
Cobalt is one of the three metals that are ferromagnetic at room temperature. It dissolves slowly in dilute mineral acids, does not combine directly with either hydrogen or nitrogen, but will combine, on heating, with carbon, phosphorus, or sulfur. Cobalt is also attacked by oxygen and by water vapour at elevated temperatures, with the result that cobaltous oxide, CoO (with the metal in the +2 state), is produced.
Natural cobalt is all stable isotope cobalt-59, from which the longest-lived artificial radioactive isotope cobalt-60 (5.3-year half-life) is produced by neutron irradiation in a nuclear reactor. Gamma radiation from cobalt-60 has been used in place of X-rays or alpha rays from radium in the inspection of industrial materials to reveal internal structure, flaws, or foreign objects. It has also been used in cancer therapy, in sterilization studies, and in biology and industry as a radioactive tracer.
Cobalt Compounds –
Common oxidation states of cobalt include +2 and +3, although compounds with oxidation states ranging from −3 to +5 are also known. A common oxidation state for simple compounds is +2 (cobalt(II)). These salts form the pink-colored metal aquo complex (Co(H2O)6)2+ in water. Addition of chloride gives the intensely blue (CoCl4) 2−. In a borax bead flame test, cobalt shows deep blue in both oxidizing and reducing flames.
Both Co2+ and Co3+ form numerous coordination compounds, or complexes. Co3+ forms more known complex ions than any other metal except platinum. The coordination number of the complexes is generally six.
One of the more important salts of cobalt is the sulfate CoSO4, which is employed in electroplating, in preparing drying agents, and for pasture top-dressing in agriculture. Other cobaltous salts have significant applications in the production of catalysts, driers, cobalt metal powders, and other salts. Cobaltous chloride (CoCl2∙6H2O in commercial form), a pink solid that changes to blue as it dehydrates, is utilized in catalyst preparation and as an indicator of humidity. Cobaltous phosphate, Co3(PO4)2∙8H2O, is used in painting porcelain and colouring glass.
Uses of Cobalt –
Cobalt has been used in the production of high-performance alloys. It can also be used to make rechargeable batteries, and the advent of electric vehicles and their success with consumers probably has a great deal to do with the DRC’s soaring production. Other important factors were the 2002 Mining Code, which encouraged investment by foreign and transnational corporations such as Glencore, and the end of the First and Second Congo Wars. In 2016, 116,000 tonnes of cobalt was used.
Most of the cobalt produced is used for special alloys. A relatively large percentage of the world’s production goes into magnetic alloys such as the Alnicos for permanent magnets. Sizable quantities are utilized for alloys that retain their properties at high temperatures and superalloys that are used near their melting points (where steels would become too soft). It is also used in lithium-ion batteries, and in the manufacture of magnetic, wear-resistant and high-strength alloys.
Cobalt is used in electroplating for its attractive appearance, hardness, and resistance to oxidation. It is also used as a base primer coat for porcelain enamels. It is used in external beam radiotherapy, sterilization of medical supplies and medical waste, radiation treatment of foods for sterilization (cold pasteurization), industrial radiography (e.g. weld integrity radiographs), density measurements (e.g. concrete density measurements), and tank fill height switches.
Information Sources: