Overview
Ciprofloxacin tablet is a broad spectrum oral antimicrobial agent which is an oral solid dosage form. Ciprofloxacin is rapidly and well absorbed from gastrointestinal tract. After oral administration Ciprofloxacin is widely distributed throughout the body. Ciprofloxacin is present in active form in the saliva, nasal and bronchial secretions, mucosa of the sinuses, sputum, peritoneal fluid, prostatic secretions. It has also been detected in lung, fat, muscle, cartilage and bone. It is mainly eliminated from the body by glomerular filtration and active tubular secretion. There is a delay in the absorption of drug when ciprofloxacin tablet is concomitantly given with food. Ciprofloxacin acts by inhibiting the enzymes topoisomerase II and topoisomerase IV, which are required for the bacterial DNA transcription, replication, repair and recombination. The microorganisms resistant to the ciprofloxacin may be occurs due to the either mutations in the DNA gyrases decreased the permeability of outer membrane or efflux mechanism of drug. So, therefore in vitro antimicrobial susceptibility testing is necessary to perform in laboratory to measure susceptibility of antibiotics of choice by various methods like dilution and/or disc diffusion method. In dilution method, the minimum inhibitory concentration of antimicrobial agents is measured by broth and/or agar dilution techniques. On the other hand, disc diffusion technique is performed by measuring the zone of inhibition. This is performed by paper disc which is impregnated with the antimicrobial agent to test the susceptibility of the microorganism to that antimicrobial agent. If the microorganism is susceptible to the antimicrobial agent that means, the agent of choice can effectively inhibit that microorganism and if the microorganism is resistant to the antimicrobial agent that means, that there is need another therapy for that microorganism.
Under the perspective of Bangladesh, which is an overpopulated country, peoples are suffered from various diseases. Antimicrobial agents like ciprofloxacin is widely used popular drug here. Treatment of the diseases can become complicated if antimicrobial resistance is developed and wrong medications are chosen to treat diseases. To overcome this situation, physicians and doctors should test the anti microbial susceptibility of selected medications and should choose the correct medications for patient. People also should careful while taking this type of medication. The purpose of this research is to determine the susceptibility of Ciprofloxacin against different species of bacteria in different brands of ciprofloxacin present in Bangladesh.
Microorganism
Microorganisms are the subject of microbiology which is the branch of science that studies microorganisms. A microorganism can be one cell or cluster of cells that can be seen only by using a microscope. Microorganisms are organized into six fields of study:
1. Bacteriology: Bacteriology is the study of bacteria which are one celled prokaryotic organisms. For example, Bacillus anthracis, is a bacterium which causes anthrax.
2. Virology: Virology is the study of viruses, which are submicroscopic, parasitic, acellular entity composed of nucleic acid core surrounded by a protein coat. For example, Varicella-Zoster virus, that causes chicken pox in humans.
3. Mycology: Mycology is the study of fungus. A fungi which is a eukaryotic organism, that absorbs nutrients from its external environment whose cells have nucleus, cytoplasm and organelles and not photosynthetic. For examples: Yeasts and some molds.
4. Phycology: Phycology is the study of algae which are eukaryotic photosynthetic microorganisms, whose cells have nucleus, nuclear envelope, cytoplasm and organelles and that is able to carry out photosynthesis.
5. Protozoology: Protozoology is the study of protozoa, animal-like single cell microorganisms that can be found in aquatic and terrestrial environments. An example is Amoeba Proteus.
6. Parasitology: Parasitology is the study of parasites which is an organism that lives at the expense of another organisms or host. Examples of parasites are: bacteria, viruses, protozoa, worms, arthropods, flatworms etc (Betsy, T. and Keogh, J.E., 2012).
A microorganism is a pathogen if it is capable of causing disease. The agents of human infectious diseases belong to five major groups of organisms: bacteria, fungi, protozoa, helminthes, and viruses (Levinson, W., 2006).
Characteristics of bacteria
- Shape and size:
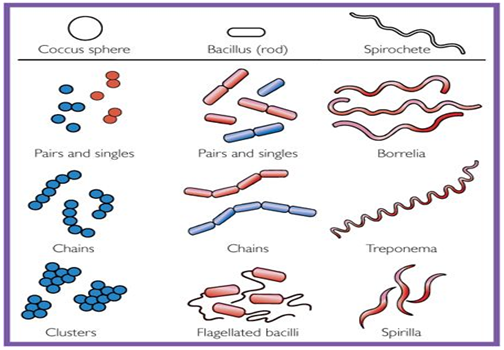
▪ Bacteria have three shapes: cocci (sphere), bacilli (rods), spirochetes (Spirals).
▪ Cocci are arranged in three patterns: pairs (diplococci), chains (streptococci), clusters (staphylococci).
▪ The size of bacteria ranges from 1μm to 3μm.
Figure 1: Shapes of Bacteria (Levinson, W., 2006).
Bacterial cell wall:
All bacteria have cell wall composed of peptidoglycan except Mycoplasma, which are surrounded by a cell membrane.
Gram-negative bacteria have thin peptidoglycan covered by an outer lipid containing membrane, whereas gram-positive bacteria have thick peptidoglycan and no outer membrane.
The outer membrane of gram-negative bacteria contains endotoxin (lipopolysaccharide).
Bacterial DNA:
▪ The bacterial genome consists of a single chromosome of circular DNA located in the nucleoid.
▪ Plasmids are extra chromosomal pieces of circular DNA that encode both exotoxins and many enzymes that cause antibiotic resistance.
▪ Transposons are small pieces of DNA that move frequently between chromosomal DNA and plasmid DNA. They carry antibiotic resistance genes.
Structures External to the Cell Wall:
▪ Capsules are antiphagocytic, they limit the ability of neutrophils to engulf the bacteria. Capsules are also antigen in several vaccines, such as pneumococcal vaccine.
▪ Pili are filaments of protein that extend from the bacterial surface and mediate the attachment of bacteria to the surface of human cells.
▪ The glycocalyx is a polysaccharide “slime layer” secreted by certain bacteria and it attaches bacteria firmly to human cells.
Gram Stain:
Gram stain is the most important staining procedure . Gram-positive bacteria have the ability to retain the crystal violet-iodine complex in the presence of lipid solvent, usually acetone-alcohol and stain purple.
Gram-negative bacteria have an outer lipid-containing membrane and thin peptidoglycan; lose the purple dye when treated with acetone-alcohol. They become colorless and then stain pink when exposed to a red dye such as safranin.
Bacterial Spores:
Spores are medically important because they are highly heat resistant are not killed by many infectants. Boiling will not kill spores.
Spores have thick, keratin like coat that allows them to survive for many years, especially in the soil.
Spores are metabolically inactive but contain DNA, ribosomes, and other essential components (Levinson, W., 2006).
Table1: Classification of bacteria (Levinson, W., 2006).
Characteristics | Genus | Representative diseases |
| I. Rigid, thick-walled cellsA. Free-living (extracellular bacteria) 1. Gram-positive a. Cocci b. Spore-forming rods (1) Aerobic (2) Anaerobic c. Non-spore-forming rods (1) Filamentous (2) Nonfilamentous 2. Gram- Negative a. Cocci b. Rods (1) Facultative (a) Straight (i) Respiratory organisms (ii) Zoonotic organisms (iii) Enteric
Characteristics |
Streptococcus Staphylococcus
Bacillus Clostridium
Actinomyces Nocardia Cornybacterium
Nesseria
Haemophilus Brucella Escherichia Enterobacter
Genus |
Pneumonia, pharyngitis Abscess of skin
Anthrax Tetanus, botulism
Actinomycosis Nocardiosis Diphtheria
Gonorrhea, meningitis
Meningitis Brucellosis UTI, diarrhea UTI
Representative Diseases |
|
(2) Aerobic (3) Anaerobic 3. Acid-fast B. Non-free living (obligate intracellular parasites) | SerratiaKlebsiella
Salmonella Shigella Pseudomonas Bacteroides Mycobacterium Rickettsia | PneumoniaUrinary tract infection, Pneumonia Enterocolitis Enterocolitis Pneumonia, UTI Peritonitis Tuberculosis, leprosy Rocky mountain spotted fever
|
| II. Flexible, thin-walled cells (Spirochetes) | Treponema | Syphilis |
| III. Wall-less cells | Mycoplasma | Pneumonia |
Antimicrobial Susceptibility Test
Bacterial strains, even from the same species, may vary widely in sensitivity to antibiotics. Information about the antimicrobial susceptibility of the infecting microorganism is important for appropriate drug selection. Several tests are available for determination of bacterial sensitivity to antimicrobial agents. The most commonly used are disc-diffusion tests, agar- or broth-dilution tests, and automated test systems (Goodman et al, 2008).
The disc-diffusion technique provides only qualitative or semi quantitative information on antimicrobial susceptibility. The test is performed by applying commercially available filter-paper discs impregnated with a specific amount of the drug onto an agar surface, over which a culture of the microorganism has been streaked. After 18 to 24 hours of incubation, the size of the clear zone of inhibition around the disc is measured. The diameter of the zone depends on the activity of the drug against the test strain. Standardized values for zone sizes for each bacterial species and each antibiotic permit classification of the clinical isolate as resistant, intermediate, or susceptible. (Goodman et al, 2008).
Dilution tests employ antibiotics in serially diluted concentrations in solid agar or broth medium containing a culture of the test microorganism. The lowest concentration of the agent that prevents visible growth after 18 to 24 hours of incubation is known as the minimal inhibitory concentration (MIC) (Goodman et al, 2008).
Automated systems also use a broth-dilution method. The optical density of a broth culture of the clinical isolate incubated in the presence of drug is determined. If the density of the culture exceeds a threshold optical density, then growth has occurred at that concentration of drug. The MIC is the concentration at which the optical density remains below the threshold (Goodman et al, 2008).
Ciprofloxacin
The first quinolone, nalidixic acid, was isolated as a by-product of the synthesis of chloroquine. It has been available for the treatment of urinary tract infections for many years. The introduction of fluorinated 4-quinolones, such as ciprofloxacin (CIPRO), represents a particularly important therapeutic advance because these agents have broad antimicrobial activity and are effective after oral administration for the treatment of a wide variety of infectious diseases (Goodman et al, 2008).
It is the most potent first generation fluoroquinolone active against a broad range of bacteria; the most susceptible ones are the aerobic Gram-negative bacilli, especially the Enterobacteriaceae and Neisseria (Tripathi et al, 2008).
Ciprofloxacin is rapidly absorbed orally, but food decays absorption and first pass metabolism occurs. The most prominent feature of ciprofloxacin is high tissue penetrability, concentration in lung, sputum, muscle, bone, prostate and phagocytes exceeds that in plasma, but CSF and aqueous levels are lower (Tripathi et al, 2008).
Molecular Formula: C17H18FN3O3, HCL
Molecular Mass: 367.8ıı
Action and use: Fluoroquinolone antibacterial.
Chemical Name: 1-Cyclopropyl-6-fluoro-4-oxo-7-(piperazin-1-yl)-1,4-dihydroquinoline-3-carboxylic acid hydrochloride.
Content
98.0 per cent to 102.0 per cent (anhydrous substance).
Characteristics
Appearance
Pale yellow, crystalline powder, slightly hygroscopic.
Solubility
Soluble in water, slightly soluble in methanol, very slightly soluble in ethanol, practically insoluble in acetone, in ethyl acetate and in methylene chloride (British pharmacopoeia, 2009).
Chemistry
Quinolones containing a carboxylic acid moiety at position 3 of the primary ring structure. Many of the newer fluoroquinolones also contain a fluorine substituent at position 6 and a piperazine moiety at position 7(Goodman et al, 2008).
Mechanism of Action of Ciprofloxacin
The quinolone antibiotics target bacterial DNA gyrase and topoisomerase IV). For many gram-positive bacteria (such as S. aureus), topoisomerase IV is the primary activity inhibited by the quinolones. In contrast, for many gram-negative bacteria (such as E. coli), DNA gyrase is the primary quinolone target. The individual strands of double-helical DNA must be separated to permit DNA replication or transcription. However, anything that separates the strands results in “overwinding” or excessive positive supercoiling of the DNA in front of the point of separation. To combat this mechanical obstacle, the bacterial enzyme DNA gyrase is responsible for the continuous introduction of negative supercoils into DNA. This is an ATP-dependent reaction requiring that both strands of the DNA be cut to permit passage of a segment of DNA through the break; the break then is resealed (Goodman et al, 2008).
The DNA gyrase of E. coli is composed of two 105,000-dalton A subunits and two 95,000-dalton B subunits encoded by the gyrA and gyrB genes, respectively. The A subunits, which carry out the strand-cutting function of the gyrase, are the site of action of the quinolones. The drugs inhibit gyrase-mediated DNA supercoiling at concentrations that correlate well with those required to inhibit bacterial growth (0.1 to 10 μg/ml). Mutations of the gene that encodes the A subunit polypeptide can confer resistance to these drugs (Goodman et al, 2008).
Topoisomerase IV also is composed of four subunits encoded by the parC and parE genes in E. coli. Topoisomerase IV separates interlinked (catenated) daughter DNA molecules that are the product of DNA replication. Eukaryotic cells do not contain DNA gyrase. However, they do contain a conceptually and mechanistically similar type II DNA topoisomerase that removes positive supercoils from eukaryotic DNA to prevent its tangling during replication. This enzyme is the target for some antineoplastic agents. Quinolones inhibit eukaryotic type II topoisomerase only at much higher concentrations (100 to 1000 μg/ml) (Goodman et al, 2008).
Pharmacokinetics of Ciprofloxacin
The goal of drug therapy is to prevent, cure, or control various disease states. To achieve this goal, adequate drug doses must be delivered to the target tissues so that therapeutic yet nontoxic levels are obtained. Pharmacokinetics examines the movement of a drug over time through the body. Pharmacological as well as toxicological actions of drugs are primarily related to the plasma concentrations of drugs. Thus, the clinician must recognize that the speed of onset of drug action, the intensity of the drug’s effect, and the duration of drug action are controlled by four fundamental pathways of drug movement and modification in the body.
First, drug absorption from the site of administration (Absorption) permits entry of the therapeutic agent (either directly or indirectly) into plasma. Second, the drug may then reversibly leave the bloodstream and distribute into the interstitial and intracellular fluids (Distribution). Third, the drug may be metabolized by the liver, kidney, or other tissues (Metabolism). Finally, the drug and its metabolites are removed from the body in urine, bile, or feces (Elimination) (Harvey et al, 2008).
Absorption
The quinolones are well absorbed after oral administration and distributed widely in body tissues. Food does not impair oral absorption but may delay the time to peak serum concentrations. Oral doses in adults are 250 to 750 mg every 12 hours for ciprofloxacin. Bioavailability of the fluroquinolones is greater than 50% for all agents and greater than 95% for several (Goodman et al, 2008).
Distribution
The volume of distributions for quinolones is high, with concentrations of quinolones in urine, kidney, lung and prostate tissue, stool, bile, macrophages and neutrophils higher than the serum levels. Quinolone concentrations in cerebrospinal fluid, bone and prostatic fluid are lower than in serum. Ciprofloxacin have been detected in human breast milk (Goodman et al, 2008).
Elimination
Most of the quinolones are cleared predominantly by the kidney and the dosages must be adjusted for renal failure (Goodman et al, 2008).
It is excreted primarily in urine, both by glomerular filtration and tubular secretion, urinary and biliary concentrations are 10-50 folds higher than plasma (Tripathi et al, 2003).
Available forms of Ciprofloxacin
Ciprofloxacin (Cipro, Cipro I.V.)
Oral: 250, 500, 750 mg tablets; 50, 100 mg/mL suspension
Parenteral: 2, 10 mg/mL for IV infusion
Ophthalmic (Ciloxan): 3 mg/mL solution; 3.3 mg/g ointment (Katzung et al 2012).
Dosage of Ciprofloxacin
By mouth: Respiratory-tract infections, 500–750 mg twice daily (750 mg twice daily in pseudomonal lower respiratory-tract infection in cystic fibrosis)
Urinary-tract infections, 250–750 mg twice daily (250 mg twice daily for 3 days usually adequate for acute uncomplicated cystitis in women)
Acute or chronic prostatitis, 500 mg twice daily for 28 days
Gonorrhoea, 500 mg as a single dose
Anthrax (treatment and post-exposure prophylaxis), 500 mg twice daily
Most other infections, 500 mg twice daily (increased to 750 mg twice daily in severe or deep-seated infection)
By intravenous infusion: Over 60 minutes, 400 mg every 8–12 hours.
Anthrax (treatment and post-exposure prophylaxis): by intravenous infusion: over 60 minutes, 400 mg every 12 hours (Thompson D, 2011).
Therapeutic Uses of Ciprofloxacin
Urinary Tract Infections
The fluoroquinolones are significantly more potent and have a much broader spectrum of antimicrobial activity for urinary tract infections caused by susceptible microorganisms. Comparative clinical trials indicate that the fluoroquinolones are more efficacious than trimethoprim-sulfamethoxazole for the treatment of urinary tract infections (Goodman et al, 2008).
Prostatitis
Ciprofloxacin have been effective in uncontrolled trials for the treatment of prostatitis caused by sensitive bacteria. Fluoroquinolones administered for 4 to 6 weeks appear to be effective in patients not responding to trimethoprim-sulfamethoxazole (Goodman et al, 2008).
Sexually Transmitted Diseases
The quinolones are contraindicated in pregnancy. Fluoroquinolones lack activity for Treponema pallidum but have activity in vitro against N. gonorrhoeae, C. trachomatis, and H. ducreyi. A single oral dose of a fluoroquinolone such as ciprofloxacin is effective treatment for sensitive strains of N. gonorrhoeae. Chancroid (infection by H. ducreyi) can be treated with 3 days of ciprofloxacin (Goodman et al, 2008).
Gastrointestinal and Abdominal Infection
For traveler’s diarrhea (frequently caused by enterotoxigenic E. coli), the quinolones are equal to trimethoprim-sulfamethoxazole in effectiveness, reducing the duration of loose stools by 1 to 3 days. Ciprofloxacin given for 5 days all have been effective in the treatment of patients with shigellosis, with even shorter courses effective in many cases. Ciprofloxacin treatment cures most patients with enteric fever caused by S. typhi, as well as bacteremic nontyphoidal infections in AIDS patients, and it clears chronic fecal carriage. Shigellosis is treated effectively with either ciprofloxacin or azithromycin (Goodman et al, 2008).
Respiratory Tract Infections
The fluoroquinolones have in vitro activity against commonly recognized respiratory pathogens, including H. influenzae, Moraxella catarrhalis, S. aureus, M. pneumoniae, Chlamydia pneumoniae, and Legionella pneumophila. Either a fluoroquinolone (ciprofloxacin or levofloxacin) or azithromycin is the antibiotic of choice for L. pneumophila. Fluoroquinolones have been very effective at eradicating both H. influenzae and M. catarrhalis from sputum. Mild to moderate respiratory exacerbations owing to P. aeruginosa in patients with cystic fibrosis have responded to oral fluoroquinolone therapy (Goodman et al, 2008).
Bone, Joint, and Soft Tissue Infections
The treatment of chronic osteomyelitis requires prolonged (weeks to months) antimicrobial therapy with agents active against S. aureus and gram-negative rods. The fluoroquinolones, by virtue of their oral administration and appropriate antibacterial spectrum for these infections, may be used appropriately in some cases; recommended doses are 500 mg every 12 hours or, if severe, 750 mg twice daily. Dosage should be reduced for patients with severely impaired renal function. Clinical cures have been as high as 75% in chronic osteomyelitis in which gram-negative rods predominat. Ciprofloxacin as sole therapy is effective in 50% of diabetic foot infections (Goodman et al, 2008).
Other Infections
Ciprofloxacin received wide usage for the prophylaxis of anthrax and has been shown to be effective for the treatment of tularemia. Ciprofloxacin plus amoxicillin-clavulanate has been shown recently to be effective as an oral empirical therapy for fever in low-risk patients with granulocytopenia secondary to cancer chemotherapy (Goodman et al, 2008).
Microbiological features of Ciprofloxacin
Remarkable features of ciprofloxacin are:
- Rapidly bactericidal activity and high potency
- Low frequency of mutational resistance
- Protective intestinal streptococci and anaerobes are spared
- Low propensity to select plasmid type resistant mutants
- Less active at acidic pH
- Relatively long post-antibiotic effect on Enterobacteriaceae, Pseudomonas and Staph.
- Active against many β-lactum and aminoglycoside resistant bacteria.
The spectrum of action is summarized below:
Highly Susceptible: ▪ E. coli ▪ Neisseria gonorrhoea
▪ K. Pneumoniae ▪ N. meningitides
▪ Enterobacter ▪ H. influenza
▪ Salmonella typhi ▪ H. ducreyi
▪ Other Salmonella ▪ Campylobacter jejuni
Moderately Susceptible: ▪ Pseudomonas aeruginosa ▪ Legionella
▪ Staphylococcus aureus ▪ Listeria
▪ Staph. Epidermidis ▪ Branhamella catarrrhalis
▪ Bacillus anthracis ▪ Mybacterum tuberculosis
Low/ variable Susceptible: ▪ Streptococcus pyogenes ▪ Streptococcus faecalis
▪ Streptococcus pneumoniae ▪ Mycobacterium Kanasii
▪ Chlamydia ▪ Mycobacterium avium
Resistant Bacteria: ▪ Bacterioides fragilis
▪ Clostridia
▪Anaerobic cocci
Side Effects of Ciprofloxacin
Ciprofloxacin has good safety record: side effects occur in ~10% patients; but are generally mild; withdrawal is needed only in 1.5%.
- Gastrointestinal: Nausea
Vomiting
Bad taste
Anorexia
Diarrhea is infrequent- because gut anaerobes are not affected.
- CNS: Dizziness
Headache
Restlessness
Anxiety
Insomnia
Impairment of concentration and dexterity
Tremor
Seizures are rare, occur only at high doses.
- Skin/Hypersensitivity: Rash
Pruritus
Phtosensitivity
Urticaria
Swelling of lips etc.
- Tendonitis and tendon rupture: A few cases have been reported (Tripathi et al, 2003).
Precautions of the use of Ciprofloxacin
General
- Quinolones should be used with caution in patients with a history of epilepsy or conditions that predispose to seizures, in G6PD deficiency, myasthenia gravis (risk of exacerbation), and in children or adolescents (arthropathy has developed in weight-bearing joints in young animals).
- Exposure to excessive sunlight should be avoided (discontinue if photosensitivity occurs).
- Quinolones may induce convulsions in patients with or without a history of convulsions; taking NSAIDs at the same time may also induce them (Thompson D, 2011)
Pregnancy
Quinolones should be avoided in pregnancy because they have been shown to cause arthropathy in animal studies; safer alternatives are available (Thompson D, 2011).
Ciprofloxacin should not be given to pregnant women (Goodman et al, 2008).
Pediatrics Use
The significance of this effect in humans is uncertain and in some specific circumstances short-term use of either ciprofloxacin may be justified in children (Thompson D, 2011).
The fluroquinolones have been contraindicated in children. Ciprofloxacin has been administered to millions of children in India and elsewhere, a few case joint pain and swelling have been reported.
Contra-indications
Renal impairment:
By mouth, 250–500 mg every 12 hours if eGFR 30–60 mL/minute/1.73m2 (every 24 hours if eGFR less than 30 mL/minute/1.73m2).
By intravenous infusion, (200 mg over 30 minutes), 200–400 mg every 12 hours if eGFR 30–60 mL/minute/1.73m2 (every 24 hours if eGFR less than 30 mL/ minute/1.73m2) (Thompson D, 2011).
Drug Interactions
- Plasma concentration of theophylline, caffeine and warfarin are increased by ciprofloxacin due to inhibition of metabolism: toxicity of this drug can occur.
- NSAIDs may enhance the CNS toxicity of fluroquinolones; seizures are reported.
- Antacids, sucralfate and iron salts given concurrently reduce absorption of fluroquinilones (Tripathi et al, 2003).
Mechanism of Ciprofloxacin Antibiotic Resistance
The recent emergence of antibiotic resistance in bacterial pathogens, both nosocomially and in the community is a very serious development that threatens the end of the antibiotic era. There now are strains of enterococci, Pseudomonas spp., and Enterobacter that are resistant to all available antibiotics. For an antibiotic to be effective, it must reach its target in an active form, bind to the target, and interfere with its function. Accordingly, bacterial resistance to an antimicrobial agent is attributable to three general mechanisms:
(1) The drug does not reach its target,
(2) The drug is not active, or
(3) The target is altered (Goodman et al, 2008).
Reduced Drug Concentration
The outer membrane of gram-negative bacteria is a permeable barrier that excludes large polar molecules from entering the cell. Small polar molecules, including many antibiotics, enter the cell through protein channels called porins. Absence of, mutation in, or loss of a favored porin channel can slow the rate of drug entry into a cell or prevent entry altogether, effectively reducing drug concentration at the target site (Goodman et al, 2008).
Mutation or phenotypic change
If the target is intracellular and the drug requires active transport across the cell membrane, a mutation or phenotypic change that shuts down this transport mechanism can confer resistance. (Goodman et al, 2008).
Efflux pump mechanism
Bacteria also have efflux pumps that can transport drugs out of the cell. Resistance to numerous drugs, including tetracycline, chloramphenicol, fluoroquinolones, macrolides, and b-lactam antibiotics, is mediated by an efflux pump mechanism depicts the multiple membrane and periplasm components that reduce the intracellular concentrations of b-lactam antibiotics and cause resistance (Goodman et al, 2008).
Alteration of target site
The third general mechanism of drug resistance is target alteration. This may be due to mutation of the natural target (e.g., fluoroquinolone resistance), target modification (e.g., ribosomal protection type of resistance to macrolides and tetracyclines), or acquisition of a resistant form of the native, susceptible target (e.g., staphylococcal methicillin resistance caused by production of low-affinity penicillin-binding protein) (Goodman et al, 2008).
Control of drug resistance
The rampant spread of antibiotic resistance mandates a more responsible approach to antibiotic use. The Centers for Disease Control and Prevention has outlined a series of steps to prevent or diminish antimicrobial resistance. Important components include:
- Appropriate use of vaccination.
- Judicious use and proper attention to indwelling catheters.
- Choosing antibiotic therapy based on local patterns of susceptibilities of organisms.
- Appropriate use of prophylactic antibiotics in surgical procedures.
- Infection control procedures to isolate the pathogen, and strict compliance to hand hygiene.
- Early involvement of infectious disease experts (Goodman et al, 2008).
Significance of the study
In the clinical microbiology laboratory an important task is the performance of antimicrobial susceptibility testing of significant bacterial isolates. The goals of testing are to detect possible drug resistance in common pathogens and to assure susceptibility to drugs of choice for particular infections. Disk diffusion method provides flexibility and possible cost savings and also provides qualitative assessments using the categories susceptible, intermediate, or resistant. In general, current testing methods provide accurate detection of common antimicrobial resistance mechanisms. The disk diffusion susceptibility method is simple and practical and has been well-standardized. The advantages of the disk method are the test simplicity that does not require any special equipment, the provision of categorical results easily interpreted by all clinicians, and flexibility in selection of disks for testing. It is the least costly of all susceptibility methods (Reller, L.B. et al 2009).
The rise of antibiotic resistance is a matter in today’s world. Multiple resistance mechanisms may be developed from antibiotic usage derived from microorganism’s genetic capacity to exploit resistance gene and horizontal gene transmission. There is need complete information on the role of microorganisms and the discovery of new antibiotics and the controlled use of antibiotic therapy. Antibiotics which are used in the treatment of infections occurred by bacterias, viruses, funguses and other parasites are must be an improved form, from the first time employed in. Antibiotics used in the treatment of diseases for human, animal, plants, fish etc occurred by any living organisms. Resistance of antibiotic occurs due to the various biochemical and physiological mechanisms. Morbidity and mortality, concern bacteria are the most striking problem today (Davies J & Davies, D., 2010).
Ciprofloxacin is active against both Gram-positive and Gram-negative bacteria. It is particularly active against Gram-negative bacteria, including salmonella, shigella, campylobacter, neisseria, and pseudomonas. Ciprofloxacin has only moderate activity against Gram-positive bacteria such as Streptococcus pneumoniae and Enterococcus faecalis; it should not be used for pneumococcal pneumonia. It is active against chlamydia and some mycobacteria. Most anaerobic organisms are not susceptible. Ciprofloxacin can be used for respiratory tract infections (but not for pneumococcal pneumonia), urinary-tract infections, infections of the gastro-intestinal system (including typhoid fever), bone and joint infections, gonorrhoea and septicaemia caused by sensitive organisms (Thompson D, 2011).
Pseudomonas aeruginosa is the most common gram-negative bacterium which is found in noscominal infections causing various infections in the body. A study was performed using subcultures of Pseudomonas aeruginosa isolates by antibacterial sensitivity test (disc diffusion techniques). Various studies conducted in Pakistan showed that, more than 90% of Pseudomonas aeruginosa isolates were sensitive to ciprofloxacin. In vitro antibiogram sensitivity studies using disc diffusion techniques was conducted 69 Pseudomonas aeruginosa isolates against 11 chemotherapeutic agents and the result showed that sensitivity of ciprofloxacin was 17.4%. Water isolates in this study were sensitive to ciprofloxacin and environment isolates were completely sensitive to ciprofloxacin. Animal and poultry isolates were completely resistant to ciprofloxacin. Human isolates were completely sensitive to ciprofloxacin (36%) (Kamel et al, 2011).
Aim of the study
The major objectives of in vitro antimicrobial susceptibility testing are-
- To measure the susceptibility of various micro-organism against Ciprofloxacin by performing susceptibility test.
- To find out the effectiveness or efficacy of different brands of Ciprofloxacin tablets available in Bangladesh.
- To compare the purity of drugs of different brands of Ciprofloxacin available in the market with the standard.
- To measure the comparative activity of standard and sample of different brands of ciprofloxacin by comparing the zone of inhibition.
Material and Method
Study design
For the in vitro antimicrobial susceptibility test of two different brands Beuflox® and Deoflox® tablet, at first three different strains of E. coli and two other different microorganisms (Pseudomons spp.and Salmonella typhi) were collected from Pathology department, Institute of Child Health and Shishu Sasthya Foundation (ICH), Mirpur-2, Dhaka. Then the clinical isolates of these microorganisms were subcultured and disc diffusion test was performed by measuring the size of zone of inhibition.
Period and place of the study
The duration of this study was six months and all the test was performed in the microbiological laboratory of East West University.
Table 2: Collected Data of Clinical isolates
Sample | Clinical Isolate |
Blood | E. coli |
Urine | E. coli |
Stool | E. coli |
Blood | Pseudomonas spp. |
Blood | Salmonella typhi |
Table 3: Details data of Beuflox and Deoflox tablet
| Name | Company | MLN | DARN | Mfg. Date | Exp. Date |
| Beuflox® | Incepta | 194&108 | 116-31-60 | July-11 | Jun-16 |
| Deoflox® | Delta | 59&175 | 048-43-60 | May-10 | May-13 |
Sample
• Beuflox®- 500 mg ciprofloxacin tablet of Incepta.
• Deoflox®- 500 mg ciprofloxacin tablet of Delta.
Materials
Names of equipments and reagents required for susceptibility testing is described in table no 4.
Table 4: Name of apparatus required for sensitivity test.
| Serial No. | Apparatus | Serial No. | Apparatus |
| 1. | Petridish | 10. | Cotton bud |
| 2. | Autoclave | 11. | Forceps |
| 3. | Laminar Air Flow | 12. | Nutrient Agar(Media) |
| 4. | Hot Air Oven | 13. | Micropipette |
| 5. | Electronic Balance | 14. | Vortex Machine |
| 6. | Ruler | 15. | Inoculating Loop |
| 7. | Beaker | 16. | Paper Disc |
| 8. | 100ml Volumetric Flask | 17. | Bunsen Burner |
| 9. | Eppendrof Tube | 18. | 1000 ml Bottle |
Table 5: Name of reagents required for susceptibility test
Serial No. | Reagents |
| 1. | Distilled water |
| 2. | Normal saline(0.9%NaCl) |
| 3. | Isopropyl alcohol |
Sample Preparation
• Two 500mg of Beuflox® and Deoflox® tablets were weighed separately and recorded in the Record Book.
• Beuflox® and Deoflox® tablets were taken separately and crushed gently in mortar and pestle. Then average weights of these two brands of tablet powder were weighed and 5mg each of them kept in different tubes.
• Distilled water was added with Beuflox® and Deoflox® powder kept in each tube to make 10ml solution.
• The solutions were mixed by shaking carefully where Beuflox® and Deoflox® readily soluble with the distilled water.
• The solutions were filtered by using filter papers. The filtrate solutions were taken another screw cap tube used for antimicrobial test.
Standard Preparation
• 5 gm of standard Ciprofloxacin powder was weighed and kept in screw cap tube.
• Then distilled water was added to tube to make 10ml of solution.
• The solution was mixed by shaking the tubes carefully where standards of Ciprofloxacin powder readily soluble with the distilled water.
Media preparation
• 5.6 gm of nutrient agar was weighed and taken in bottle and mixed with 200ml of distilled water.
• Then the solution was mixed vigorously to make a homogenous mixture.
• The mixture was kept into an autoclave for a certain period of time under specific conditions of sterilization.
Preparation of dried filter paper discs
Whatman filter paper no. 1 is used to prepare discs approximately 6 mm in diameter, which are placed in a Petri dish and sterilized in hot air oven before use to the sensitivity test.
Preparation of Agar plate
1. An agar plate is a Petri dish that contains a growth medium (typically agar plus nutrients) used to culture microorganisms.
2. Agar plate was allowed to come to room temperature.
3. The agar plate was kept in laminar air flow.
4. If the agar present on the lid of the agar plate that means agar has excess liquid on the plate.
5. Each agar plate was appropriately labeled for each microorganism to be tested.
Antibiotic and blank disc placement on the agar plate
1. Selective growth compounds may also be added to the media, such as antibiotics.
2. A parameter marker was used to marks the bottoms of the agar plates with sections according to the number of antibiotic before the placement of Ciprofloxacin antibiotic disc in the test agar plate. The sections were numbered the sequentially.
3. The disc was pressed with the forceps to ensure complete contact with agar surface. The lid of the plate was replaced between discs to minimize exposure to air borne contaminants.
4. Blank disc was removed from the cartridge using forceps that had been sterilized. The plate was lifted and the disc was placed over one of the positioning marks.
Incubation of the plates
1. Then the plates were incubated within 15 minutes after applying the disks.
2. The temperature range of 350+20C is normally required for incubation and the incubation time was 24 which were considered as standard for this test.
3. If the incubation period is extended for slow-growing organisms, the stability of the agent over the incubation period must be assessed by the inclusion of control strains with known MICs.
4. Avoid incubation in an atmosphere containing 5% CO2 unless necessary for growth of an organisms.
5. Results were read after 24 hours of incubation.
Measuring the size of the zones
1. Individual microorganisms placed on the plate will grow into individual colonies, each a clone genetically identical to the individual ancestor organism.
2. The clear region around the paper disc saturated with an antimicrobial agent on the agar surface.
3. Measure the diameter of the zone of inhibition around each disk.
4. Keeping the lid of the plate in place, using a ruler to measure the diameter of the clear area in millimeters.
5. Following incubation, the zone sizes were measured to the nearest millimeter using a ruler. The diameter of the disc was included in the measurement.
6. If the placement of the disc or the size of the zone did not allow to read the diameter of the zone, then it was measured from the centre of the disc to a point on the circumference of the zone where a distinct edge was present (the radius) and the measurement was multiplied by 2 to determine the diameter.
7. The zone size was recorded on the recording sheet.
8. The lowest concentration of an antimicrobial agent that will inhibit the visible growth of a microorganism is known as the MIC.
2.5.9 Safe Disposal of Plates
1. At the conclusion of the experiment, all plates should be disinfected for safe disposal.
2. The best way to dispose of bacterial cultures is to autoclave them and cleans them properly.
3. If autoclave is not available, an alternative is to bleach the plates.
- Proper safety equipment (gloves, lab coat, and eye protection) must be need to wear when working with the bleach solution; it is corrosive.
- Saturate the plates with a 20% household bleach solution (in other words, one part bleach and four parts water).
- Allow the plates to soak overnight in the bleach solution before disposing of them.
Results
Antimicrobial susceptibility test was performed for two different antibiotics Beuflox® – 500 mg and Deoflox® – 500 mg for standard Ciprofloxacin solution using two different strains of E. coli and two other microorganisms (Pseudomonas spp. and Salmonella typhi).
Data of zone of inhibition of 5μg/disc for standard against E. coli
Table 6: Data of zone of inhibition of 5μg/disc for standard against E. coli
Clinical Isolate | Zone of Inhibition (mm) |
Blood | 20.5 |
Urine | 13 |
Stool | 20 |
The data shows that, the diameter of the zone of inhibition of Ciprofloxacin standard of three different strains of E. coli isolates collected from blood, urine and stool were 20.5 mm, 13 mm and 20 mm respectively. The blood sample and stool sample was susceptible to Ciprofloxacin standard but the urine sample was resistant to the standard.
Data of zone of inhibition of 5μg/disc for standard against Pseudomonas spp.
Table 7: Data of zone of inhibition of 5μg/disc for standard against Pseudomonas spp.
| Standard | Clinical Isolates | Zone of Inhibition (mm) |
Blood | 40 |
The data shows that, the diameter of the zone of inhibition of Ciprofloxacin standard against Pseudomonas spp. isolates collected from blood sample was 40 mm. Blood sample of Pseudomonas spp. isolates was susceptible to standard.
Data of zone of inhibition of 5μg/disc for standard against Salmonella typhi
Table 8: Data of zone of inhibition of 5μg/disc for standard against Salmonella typhi
| Standard | Clinical Isolates | Zone of Inhibition (mm) |
Blood | 40 |
The data shows that, the zone of inhibition of ciprofloxacin standard against Salmonella typhi isolates collected from blood sample was 40 mm. Blood sample of Salmonella typhi isolates was susceptible to standard.
Data of zone of inhibition of 5μg/disc for Beuflox tablet against E. coli
Table 9: Data of zone of inhibition of 5μg/disc for Beuflox tablet against E. coli
Sample | Name of the microorganism | Clinical Isolates | Zone of Inhibition (mm) |
| Beuflox Tablet | E.coli | Blood | 12 |
The data shows that, the zone of inhibition of Beuflox tablet against E.coli isolates collected from blood sample was 12 mm. Blood sample of E.coli isolates was resistant to brand Beuflox tablet.
Data of zone of inhibition of 5μg/disc for Beuflox® tablet against E. coli
Table 10: Data of zone of inhibition of 5μg/disc for Beuflox® tablet against E. coli
Sample | Name of the microorganism | Clinical Isolates | Zone of Inhibition (mm) |
| Beuflox® Tablet | E. coli | Stool | 17 |
The data shows that, the zone of inhibition of Beuflox® Tablet against E. coli isolates collected from blood sample was 17 mm. Stool sample of E. coli isolates was intermediate to Beuflox tablet.
2.6.6 Data of zone of inhibition of 5μg/disc for Beuflox® tablet against E. coli
Table 11: Data of zone of inhibition of 5μg/disc for Beuflox® tablet against E. coli
Sample | Name of the microorganism | Clinical Isolates | Zone of Inhibition (mm) |
| Beuflox® Tablet | E. coli | Urine | 20 |
The data shows that, the zone of inhibition of Beuflox® Tablet against E.coli isolates collected from urine sample was 20 mm.
Data of zone of inhibition of 5μg/disc for Beuflox® tablet against Pseudomonas spp.
Table 12: Data of zone of inhibition of 5μg/disc for Beuflox® tablet against Pseudomonas spp.
Sample | Name of the microorganism | Clinical Isolates | Zone of Inhibition (mm) |
| Beuflox® Tablet | Pseudomonas spp. | Blood | 40 |
The data shows that, the zone of inhibition of Beuflox® Tablet against Pseudomonas spp. isolates collected from blood sample was 40 mm.
Data of zone of inhibition of 5μg/disc for Beuflox® tablet against Salmonella typhi
Table 13: Data of zone of inhibition of 5μg/disc for Beuflox® tablet against Salmonella typhi
Sample | Name of the microorganism | Clinical Isolates | Zone of Inhibition (mm) |
| Beuflox® Tablet | Salmonella typhi | Blood | 39 |
The data shows that, the zone of inhibition of Beuflox® Tablet against Salmonella typhi isolates collected from blood sample was 39 mm.
Data of zone of inhibition of 5μg/disc for Deoflox® tablet against E.coli
Table 14: Data of zone of inhibition of 5μg/disc for Deoflox tablet against E.coli
Sample | Name of the microorganism | Clinical Isolates | Zone of Inhibition (mm) |
| Deoflox® Tablet | E.coli | Blood | 12 |
The data shows that, the zone of inhibition of Deoflox® Tablet against E.coli isolates collected from blood sample was 12 mm.
Data of zone of inhibition of 5μg/disc for Deoflox® tablet against E. coli
Table 15: Data of zone of inhibition of 5μg/disc for Deoflox® tablet against E. coli
Sample | Name of the microorganism | Clinical Isolates | Zone of Inhibition (mm) |
| Deoflox® Tablet | E.coli | Urine | 20 |
The data shows that, the zone of inhibition of Deoflox® Tablet against E. coli isolates collected from urine sample was 20 mm.
Data of zone of inhibition of 5μg/disc for Deoflox® tablet against E. coli
Table 16: Data of zone of inhibition of 5μg/disc for Deoflox® tablet against E. coli
Sample | Name of the microorganism | Clinical Isolates | Zone of Inhibition (mm) |
| Deoflox® Tablet | E. coli | Stool | 18 |
The data shows that, the zone of inhibition of Deoflox® Tablet against E. coli isolates collected from stool sample was 18 mm. Stool sample of E. coli isolates was intermediate to the Deoflox® tablet.
Data of zone of inhibition of 5μg/disc for Deoflox® tablet against Pseudomonas spp.
Table 17: Data of zone of inhibition of 5μg/disc for Deoflox® tablet against Pseudomonas spp.
Sample | Name of the microorganism | Clinical Isolates | Zone of Inhibition (mm) |
| Deoflox Tablet | Pseudomonas spp. | Blood | 38 |
The data shows that, the zone of inhibition of Deoflox® Tablet against Pseudomonaspp.s isolates collected from blood sample was 38 mm. Blood sample of Pseudomonas spp. isolates was susceptible to the Deoflox® tablet.
Data of zone of inhibition of 5μg/disc for Deoflox® tablet against Salmonella typhi
Table 18: Data of zone of inhibition of 5μg/disc for Deoflox® tablet against Salmonella typhi
Sample | Name of the microorganism | Clinical Isolates | Zone of Inhibition (mm) |
| Deoflox® Tablet | Salmonella typhi | Blood | 39 |
The data shows that, the zone of inhibition of Deoflox® Tablet against Salmonella typhi isolates collected from blood sample was 39 mm. Blood sample of Salmonella typhi isolates was susceptible to the Deoflox® tablet.
Comparison the zone of inhibition of sample (Beuflox® and Deoflox® Tablets) and Standard
Comparison the zone of inhibition of sample (Beuflox® and Deoflox® Tablets) and Standard against E. coli isolates collected from stool sample
Figure 7: Comparison the zone of inhibition of sample (Beuflox® and Deoflox® Tablets) and Standard against E. coli isolates collected from stool sample
This figure shows that, the diameter of the zone of inhibition of standard Ciprofloxacin was 20 mm; whereas the diameter of the zone of inhibition of Deoflox® tablet was 18 mm, where the zone of inhibition of Beuflox® tablet was 17 mm.
Comparison the zone of inhibition of sample (Beuflox® and Deoflox® Tablets) and Standard against E. coli isolates collected from blood sample
Figure 8: Comparison the zone of inhibition of sample (Beuflox® and Deoflox® Tablets) and Standard against E. coli isolates collected from blood sample.
This figure shows that, the zone of inhibition of standard Ciprofloxacin was 13 mm; whereas the zone of inhibition of Deoflox® tablet was 12 mm and the diameter of the zone of inhibition of Beuflox® tablet was 12 mm.
Comparison the zone of inhibition of sample (Beuflox® and Deoflox® Tablets) and Standard against E. coli isolates collected from urine sample
Figure 9: Comparison the zone of inhibition of sample (Beuflox® and Deoflox® Tablets) and Standard against E. coli isolates collected from urine sample
This figure shows that, the zone of inhibition of standard Ciprofloxacin was 20.5 mm; whereas the zone of inhibition of Deoflox® tablet was 20 mm, providing similar result against E. coli isolates collected from urine sample compared to the Beuflox® tablets where the diameter of the zone of inhibition of Beuflox® tablet was 20 mm.
Comparison the zone of inhibition of sample (Beuflox® and Deoflox® Tablets) and Standard against Pseudomonas spp. isolates collected from blood sample
Figure 10: Comparison the zone of inhibition of sample (Beuflox and Deoflox Tablets) and Standard against Pseudomonas spp. isolates collected from blood sample
This figure shows that, the diameter of the zone of inhibition of standard Ciprofloxacin was 13 mm; whereas the diameter zone of inhibition of Deoflox® tablet was 38 mm, providing reduced susceptibility against Pseudomonas spp. isolates compared to the Beuflox® tablets where the diameter of the zone of inhibition of Beuflox® tablet was 40 mm.
Comparison the zone of inhibition of sample (Beuflox® and Deoflox® Tablets) and Standard against Salmonella typhi isolates collected from blood sample
Figure 11: Comparison the zone of inhibition of sample (Beuflox® and Deoflox® Tablets) and Standard against Salmonella typhi isolates collected from blood sample
This figure shows that, the diameter of the zone of inhibition of standard Ciprofloxacin was 40 mm; whereas the diameter zone of inhibition of Deoflox® tablet was 39 mm, providing similar susceptibility against Salmonella typhi isolates compared to the Beuflox® tablets where diameter of the zone of inhibition of Beuflox® tablet was 39 mm.
Discussion & Conclusion
Bangladesh is a developing country where there is more chance to drug resistance occur than the developed countries due to lack of education, lack of hygiene, lack of monitoring the authorities of drug regulatory control, low standard of life, irrational and substandard use of drug. Thus we should be concerned about quality of drugs available in the market.
Widespread use and abuse of antibiotics results in an antibiotic resistance which is a global problem in humans. Because of the concentration of susceptible patients and high antibiotic selection pressure, now the problem of antibiotic-resistant bacterial infections is especially important in health care facilities (Wong et al, 2007).
This study was performed by using two different brands of Ciprofloxacin like Beuflox®, manufactured by Incepta Pharmaceutical Company & limited and Deoflox®, manufactured by Delta Pharmaceutical Company & limited. The zone of inhibition of the drug sample was compared with the zone of inhibition of the Ciprofloxacin standard. Compared to the Ciprofloxacin standard, results of this experiment showed that both Beuflox® – 500 mg and Deoflox® – 500 mg have similar susceptibity to E. coli isolated from urine sample, Pseudomonas spp. and Salmonella typhi. A study was performed where the zone of inhibition of ≤30 mm detected salmonella typhi isolates with a ciprofloxacin MIC of ≥0.125 μg/ml with a sensitivity of 94.0% and specificity of 94.2% (Christofer et al, 2010). Asna et al (2003) reported that, S. typhi isolates were fully susceptible to 5 μg ciprofloxacin (zone of inhibition >21mm). In this study, two brands Beuflox® and Deoflox® also shown similar susceptibilty (zone of inhibtion >30) compared to the standard. Quality of pharmaceutical product is very important because drugs must be marketed as safe. If the drug is not pure then it will not able to reach the plasma level required for therapeutic activity. Thus the microorganisms can become tolerated at the low concentration of antibiotic. Ciprofloxacin is a broad-spectrum antibiotic widely prescribed in clinical and hospital settings. The emergence of antimicrobial resistance against effective antibiotics is a global issue. Antimicrobial resistance surveillance is becoming critical in a global situation of increased occurrence and spread of resistance genes among bacterial pathogens. Continuous surveillance is crucial to monitor the antimicrobial resistance among pathogens.
References
Asna, S. M., Haq, J. A. & Rahman, M., 2003 . Nalidixic acid-resistant Salmonella enterica serovar typhi with decreased susceptibility to ciprofloxacin caused treatment failure: a report from Bangladesh. Jpn J Infectious Diseases 56, pp.32–33.
Betsy, T. and Keogh, J.E., 2012. Microbiology demystified. 2nd ed. McGrow-Hill companies. pp. 4-5.
British Pharmacopoeia Commission, 2009. British Pharmacopoeia. Monographs: Medicinal and Pharmaceutical Substances. p. 1381.
Cardoso, A.M. Junqueira-Kipnis, A.P. Kipnis, A., 2010. In Vitro Antimicrobial Susceptibility of Mycobacterium massiliense Recovered from Wound Samples of Patients Submitted to Arthroscopic and Laparoscopic Surgeries.
Christopher M. P., Chau T. T., Sabina D., 2010. Antimicrobial agents of chemotherapy. Suitable Disk Antimicrobial Susceptibility Breakpoints Defining Salmonella enterica Serovar Typhi Isolates with Reduced Susceptibility to Fluoroquinolones . vol.54(12), pp.5201–5208
Davies, J. Davies, D., 2010. Origins and Evolution of Antibiotic Resistance. American Society for Microbiology and Molecular Biology Reviews, 74(3), pp. 417-433.
Gilman, A.G. Hardman, J.G. Limbird L.E., 2008. The Pharmacological Basis Of Therapeutics. United States of America: The McGraw-Hill Companies, pp. 1095-1100, 1119, 1121-1122.
Harvey, R.A. Champe, P.C. Finkel, R. Cubeddu, L. Clarke, M.A., 2008. Lippincott’s Illustrated Reviews: Pharmacology. 4th ed. Lippincott Williams & Wilkins. p. 1, 388.
Kamel, G.M. Eldeen, N.A.E. Mishad, M.Y.E. Ezzat, R.F., 2011. Susceptibility Pattern of Pseudomonas aeruginosa Against Antimicrobial Agents and Some Plant Extracts with Focus on its Prevalence in Different Sources. pp. 61-72.
Katzung, B.G. Masters, S.B. Trevor, A.J., 2012. Basic & Clinical Pharmacology. 12th ed. pp. 1087-1088.
Levinson, W., 2006. Medical Microbiology & Immunology. 8th ed. The McGraw-Hill companies. pp. 1, 4-12, 24.
Rang, H.P. Dale, Maureen, M. Ritter, J.M., 2007. Rang and Dale’s Pharmacology. 6th ed. Elsevier Science Health Science div. p. 673.
Reller, L.B. Weinstein, Jorgensen J.H. Ferraro M.J., 2009. Antimicrobial Susceptibility Testing: A Review of General Principles and Contemporary Practices. Clinical Infectious Dieseases, 49 (11), pp.1749-1755.
Thompson D., 2011. British National Formulary,BNF 61. Royal Pharmaceutical Society and Pharmaceutical Press. London, UK. pp. 368-369.
Tripathi, K.D., 2008. Essentials of Medical Pharmacology. 6th ed. New Delhi, India: Jaypee Brothers Medical Publishers (P) Ltd. pp. 688-689.
Wong, S.M.Y., Ho, P.L., Yuen Y.K., 2007. Peritoneal Dialysis International. Evoluation of antibiotic resistance mechanisms and their relevance to dialysis-related patients, volume 27.