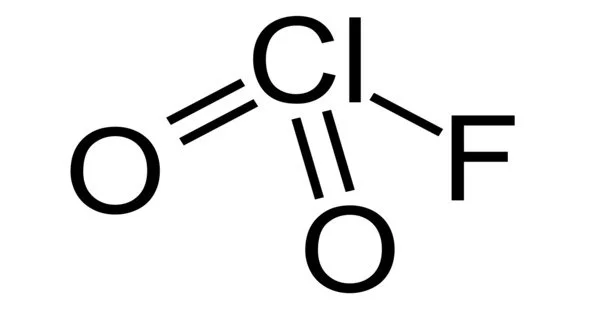
Chloryl fluoride is a chemical compound with the formula ClO2F. It is a common byproduct of chlorine fluoride reactions with oxygen sources. It is the chloric acid acyl fluoride. It is any of several organic compounds made up of carbon, fluorine, and chlorine. When CFCs contain hydrogen in place of one or more chlorines, they are referred to as hydrochlorofluorocarbons, or HCFCs.
Properties
- Chemical formula: ClFO2
- Molar mass: 86.45 g·mol−1
- Density: 3.534 g/L
- Melting point: −115 °C
- Boiling point: −6 °C
Chemical properties
Chlorine trifluoride is a very reactive compound, consequently it can suffer a different kind of reaction such as: the formation of hydrogen fluoride and hydrogen chloride through the controlled reaction with water:
ClF3 + 2H2O → 3HF + HCl + O2
ClF3 + H2O → HF + HCl + OF2
Phosphorus trichloride and phosphorus pentafluoride are formed as a result of reactions with phosphorus. Similarly, the reaction with sulfur can result in the formation of sulfur dichloride and sulfur tetrafluoride.
Preparation
Schmitz and Schumacher reported ClO2F for the first time in 1942, when they fluorinated ClO2. The compound is more easily prepared by treating sodium chlorate and chlorine trifluoride and purifying it through vacuum fractionation, which is the process of selectively condensing this species from other products. This species is a gas with a boiling point of – 6 degrees Celsius:
6 NaClO3 + 4 ClF3 → 6 ClO2F + 2 Cl2 + 3 O2 + 6 NaF
Structure
ClO2F is a pyramidal molecule, as opposed to O2F2. VSEPR foresees this structure. The differences in structure reflect chlorine’s proclivity to exist in positive oxidation states with oxygen and fluorine ligands. Perchloryl fluoride, ClO3F, is a related Cl-O-F compound. Under standard conditions, the related bromine compound bromyl fluoride (BrO2F) has the same structure as ClO2F, whereas iodyl fluoride (IO2F) forms a polymeric substance.
Uses
It is used to clean semiconductors from chemical deposition in the electronic industry. It was investigated as a chemical weapon during World War II. It is used in the fabrication of nuclear fuel.
Health effects/safety hazards
It is poisonous to humans and extremely hazardous to the environment and animals. Long-term exposure causes serious health problems. It is highly reactive. Organic and inorganic compounds can easily cause it to explode. It is highly corrosive to metals and violently reacts with hot water.