Cement is the general term given to the powdered materials which initially have plastic flow when mixed with water or other liquid but has property of setting to a hard solid structure in several hours with varying degree of strength and bonding properties. Cement is one of the most important building materials at the present time.
Cements are mainly of two types.
- Hydraulic cement
- Non-hydraulic cement

Hydraulic cement is one which requires water to attain adhesive properties, where as the non-hydraulic cement does require water for getting adhesive properties e.g. araldite, fevicol etc. hydraulic cements are also two types. They are natural and Portland cement.
- Natural cement : A hydraulic cement produced by calcining a naturally occurring argillaceous lime stone at a temperature below the sintering point and grinding to a fine powder.
- Portland cement : Hydraulic cement produced by pulverizing clinker consisting essentially of hydraulic calcium silicates, usually containing one or more of the forms of calcium sulfate as an underground addition, hydraulic calcium silicates posses ability to harden without drying or by reaction with atmospheric carbon dioxide. The reactions involved in the hardening of cement are hydration and hydrolysis. Its hardening is due to chemical reaction between the cement water.
Non-hydraulic cement: The carbonation reaction requires the dry cement to be exposed to air, and for this reason the slaked lime is a non-hydraulic cement and cannot be used under water. This whole process is called the lime cycle.

The reactions are given below:
- Reactions of tricalcium silicate (C3S) with H2O.
2(3CaO.SiO2) + 6H2O → 3CaO.SiO2.3H2O + 3Ca(OH)2
- Reactions of dicalcium silicates (C2S) with H2O.
2(2CaO.SiO2) + 4H2O → 3Ca.2SiO2. 3H2O + Ca(OH)2
- Reaction of tricalcium aluminate (C3A) with H2O.
3CaO. Al2O3 + 6H2O → 3CaO.Al2O3. 6H2O
- Reaction of tetra calcium aluminates ferrite (C4AF) with H2O.
4CaO.Al2O3. Fe2O3 + 2Ca (OH)2 + 10H2O → 3CaO. Al2O3. 6H2O + 3CaO. Fe2O3. 6H2O
- Reaction of tridicalcium aluminates (C3A), Gypsum with H2O.
- Reaction of uncombined lime (CaO) magnesium oxide (MgO) with H2O.
CaO + 06. Reaction of uncombined lime (CaO) and Magnesium oxide (MgO) with H2
CaO+H2O → Ca(OH)2 and MgO + H2O → Mg(OH)2
7. Reaction of sodium and potassium oxide with H2O.
Na2O + H2O → 2NaOH and K2O + H2O → 2KOH
This leads to the insoluble product. When used in concrete or mortar, it binds to the aggregate together into a solid mass.
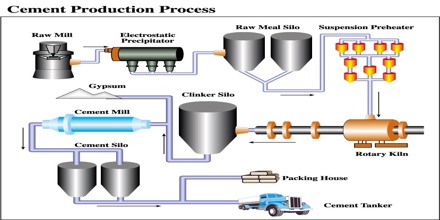
Methods for manufacturing cement:
Cement is generally manufactured by the three processes given below:
- Wet process
- Dry process
- Semidry process
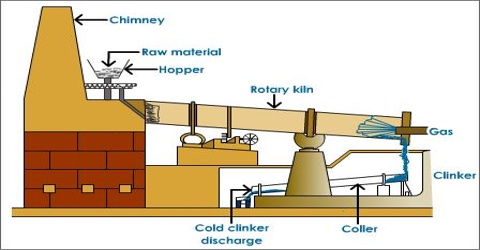
Wet process: Wet Process is most common and is almost universally employed for the manufacture of cement. In this process the raw materials are finely group blended in the designed portion and the mix is brought to the condition of free flowing slurry containing 30-40% water. The slurry is thoroughly homogenized with the help of compressed air and introduced into a rotary kiln. The change slowly moves down the kiln due to the rotary motion while a blast of burning coal is blown up from the other end of the kiln.
Dry process: In this process, the calcareous argillaceous materials are crashed in gyratory crushers to small pieces, dried and mixed in proper proportion, pulverized, the tube mills and homogenized in mixing mill with the help of compressed air. This “raw meal” is introduced into the upper of the rotary kiln while a blast of burning coal dust is blown from the other end. The reaction taking place and the rest of the process is same as described in wet process.
Semi dry process: in this process, the raw materials are good dry, but instead of feeding as a powder the “raw meal” is nodules with ID-40% water in pan or drum type nebulizer.