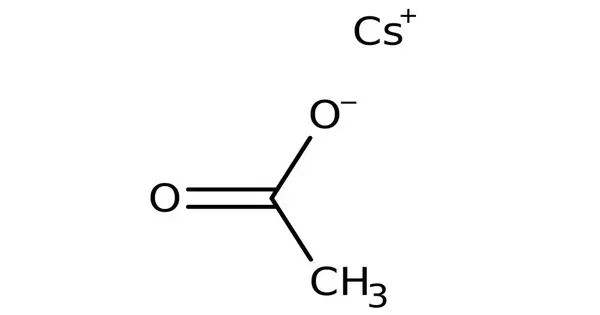
Caesium acetate or cesium acetate is an ionic caesium compound with the molecular formula CH3COOCs. It is the caesium salt of acetic acid and is typically found as a white crystalline solid. It is a white solid that may be formed by the reaction of caesium hydroxide or caesium carbonate with acetic acid. It is the cesium salt of acetic acid and exhibits both ionic and covalent properties.
Properties
Caesium acetate is a white, crystalline solid at room temperature. It is hygroscopic, meaning it readily absorbs water from the air. It is highly soluble in water. It can also dissolve in polar organic solvents like ethanol. It is relatively stable in normal conditions but can react under extreme conditions, especially with acids, forming caesium salts.
- Chemical formula: C2H3CsO2
- Molar mass: 191.949 g/mol
- Appearance: colourless, hygroscopic
- Density: 2.423 g/cm3, solid
- Melting point: 194 °C (381 °F; 467 K)
- Boiling point: 945 °C (1,733 °F; 1,218 K)
- Solubility in water: 945.1 g/100 g (−2.5 °C), 1345.5 g/100 ml (88.5 °C)
Natural Occurrence
Caesium acetate does not naturally occur in large quantities in the earth’s crust. However, it is derived from the reaction of caesium salts with acetic acid, and caesium itself is typically extracted from minerals such as pollucite, a rare mineral that contains caesium.
Industrial and Laboratory Synthesis
Caesium acetate is typically prepared in the laboratory by neutralizing acetic acid with caesium hydroxide (CsOH):
CsOH + CH₃COOH → CsC₂H₃O₂ + H₂O
It is also produced through the interaction of caesium carbonate with acetic acid.
Uses
It is used in organic synthesis. One example is in the Perkin synthesis: the formation of unsaturated cinnamic-type acids by the condensation of aromatic aldehydes with fatty acids. Replacement of the commonly used sodium acetate with caesium acetate has been shown to improve yields by up to 10 times.
It is often used to invert secondary alcohols. After converting the alcohol to a good leaving group, such as a mesylate, direct SN2 substitution with the acetate produces the O-acetate with inverted stereochemistry, which can be converted back to a hydroxyl group.
Safety
Like other caesium compounds, caesium acetate can be reactive and should be handled with caution. It can react with water to produce heat and caesium hydroxide, which is a strong base.