Type 1 diabetes is an autoimmune disease that destroys the pancreas’ insulin-producing beta cells. The body cannot regulate blood sugar levels properly without insulin, which can lead to a variety of health complications. Recently, scientists developed an efficient method for transplanting pancreatic islets and demonstrated that the method can effectively reverse type 1 diabetes in nonhuman primates.
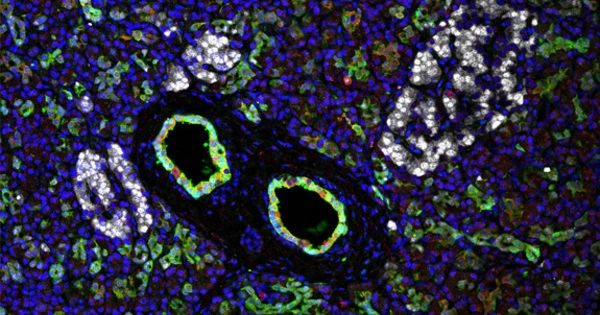
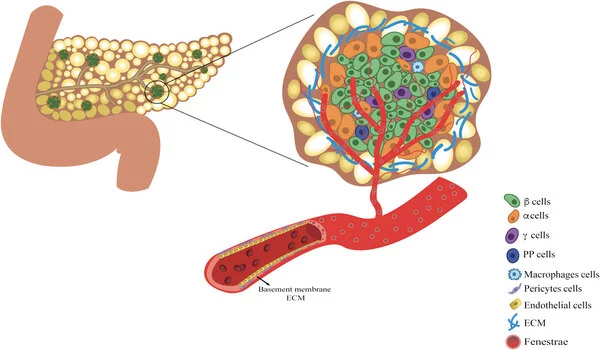
In people with type 1 diabetes, the immune system attacks and destroys insulin-producing β cells, which are part of a group of cells in the pancreas called pancreatic islets. A team led by investigators at Massachusetts General Hospital (MGH), a founding member of Mass General Brigham, recently developed an efficient way to transplant pancreatic islets and demonstrated that the method can effectively reverse type 1 diabetes in nonhuman primates in a study published in Cell Reports Medicine.
Pancreatic islet transplantation is a promising treatment option for type 1 diabetes; however, current methods, which involve transplanting islets to the liver, are inefficient and can result in the loss of up to half of transplanted cells due to immune attack. Furthermore, the liver can only hold a certain amount of transplanted tissue. Scientists have speculated that a different location might provide a more hospitable environment and yield better results. The omentum, the fatty tissue that begins in the stomach and drapes over the intestines, is one promising site.
This pre-clinical study can inform the development of new strategies for β cell replacement in diabetes and could change the current paradigm of clinical pancreatic islet transplantation.
Ji Lei
To optimize the omentum as a transplant site in an individual, researchers used topical recombinant thrombin (which stops bleeding), an enzyme, and the recipient’s own plasma to create a bio-degradable matrix that immobilizes donor islets onto the omentum. When combined with immunosuppressive therapy to protect islets from immune attack, this strategy normalized blood glucose levels and restored glucose-responsive insulin secretion in three nonhuman primates with type 1 diabetes for the duration of the study.
“The achievement of complete glycemic control is attributed to the bioengineering approach that facilitates the process of revascularization and reinnervation for the transplanted islets,” says first author Hong Ping Deng, MD, MSc, a researcher of Transplant Surgery at MGH. “which is the first time that such a demonstration has been made in a nonhuman primate model.”
“This pre-clinical study can inform the development of new strategies for β cell replacement in diabetes and could change the current paradigm of clinical pancreatic islet transplantation,” says senior corresponding author Ji Lei, MD, MBA, MSc, a principal physician investigator of Transplant Surgery at MGH and an assistant professor of Surgery at Harvard Medical School. “A clinical trial is being planned to test this approach.”
Lei, who is also the director of MGH’s Human Islet/Cell Processing Special Service cGMP Facility, points out that, in addition to transplanting islets from donors, researchers are also investigating the potential broad application of transplanting stem cell-derived islets, which cured a patient with type 1 diabetes for the first time in human history in 2022 and could provide an infinite supply of transplantable tissue. However, there are some concerns about this approach, including the possibility of tumor development.
Unlike the liver, the omentum is easily accessible for monitoring, and its non-vital site status allows for the removal of transplanted tissue in the event of complications, with either stem cell-derived islets or donor islets. Furthermore, the engineered omental site can house a variety of other genetically engineered cells, particularly for liver-based or inherited metabolic or endocrine disorders.