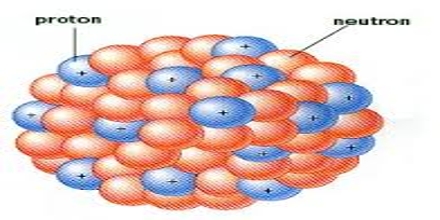
The particular atomic nucleus was discovered in 1911 by Ernest Rutherford, 1909 Geiger–Marsden gold foil experiment. Bulk of the mass within an atom comprises from the protons and neutrons in the nucleus with small contribution on the orbiting electrons. Neutrons don’t have any charge and protons usually are positively charged. Because the nucleus is only composed of protons and neutrons it really is positively charged.
Atomic Nucleus