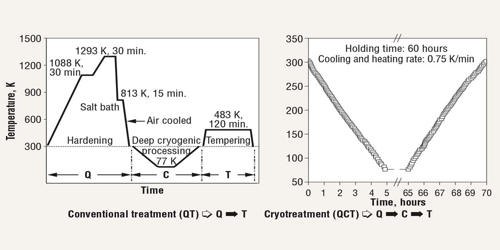
Cryogenic hardening is a cryogenic treatment process where the material is cooled to approximately −185 °C (−301 °F), usually using liquid nitrogen. It is a unique metal treatment process in which metal is intentionally exposed to extremely cold temperatures. It is able to make metal objects and workpieces more resistant to wear and tear. It can have a profound effect on the mechanical properties of certain steels, provided their composition and prior heat treatment are such that they retain some austenite at room temperature. While temperatures vary, it’s not uncommon for metal to reach -301 degrees Fahrenheit during this process. It is designed to increase the amount of martensite in the steel’s crystal structure, increasing its strength and hardness, sometimes at the cost of toughness.
Cryogenic hardening is a metal treatment process that’s characterized by the use of liquid nitrogen to freeze metal. Presently this treatment is being practiced over tool steels, high-carbon, high-chromium steels, and in some cases to cemented carbide to obtain excellent wear resistance. Metals like steel, iron, copper, and aluminum are often heat-treated to improve their physical properties. Recent research shows that there is a precipitation of fine carbides (eta carbides) in the matrix during this treatment which imparts very high wear resistance to the steels. When metal is exposed to heat, it undergoes a chemical reaction in which its atoms expand. Cryogenic hardening, however, can eliminate these stresses to achieve a uniform composition.
Cryogenic hardening treatments provide increased stress relief to metals in the manufacturing of parts and components for a wide range of applications. To perform cryogenic hardening, metal is first exposed to heat using a conventional heat-treatment process. Next, the metal is slowly cooled using liquid nitrogen. The transformation from austenite to martensite is mostly accomplished through quenching, but in general, it is driven farther and farther toward completion as temperature decreases. In higher-alloy steels such as austenitic stainless steel, the onset of transformation can require temperatures much lower than room temperature.
Cryogenic stress relief increases the overall durability and strength of a material, leading to improved part performance and prolonged life expectancy. More commonly, an incomplete transformation occurs in the initial quench, so that cryogenic treatments merely enhance the effects of prior quenching. However, since martensite is a non-equilibrium phase on the iron-iron carbide phase diagram, it has not been shown that warming the part after the cryogenic treatment results in the re-transformation of the induced martensite back to austenite or to ferrite plus cementite, negating the hardening effect. While it’s most commonly performed on steel, it can be performed on iron, copper, aluminum, magnesium, and other metals as well.