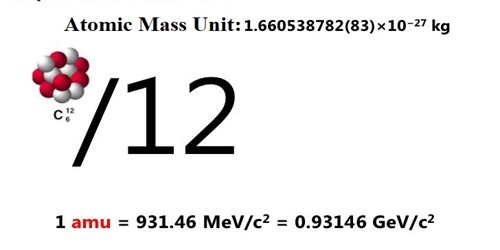
An atomic mass unit (abbreviated: amu, u, or Da) is a unit of measurement that is used to measure the mass of atoms. The atomic mass unit is equal to the 1⁄12 of the mass of the carbon-12. It is defined as precisely 1/12 the mass of an atom of carbon-12. The carbon-12 (C-12) atom has six protons and six neutrons in its nucleus. It is a unit of mass used to express atomic masses and molecular masses. The mass of any isotope of any element is expressed in relation to the carbon-12 standard.
John Dalton first suggested a means of expressing relative atomic mass in 1803. The unified atomic mass unit and the Dalton are different names for the same thing. The Dalton name is used more over time. The unit is named after John Dalton, an 18th-century naturalist and teacher.
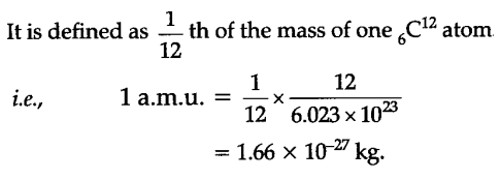
The symbol for the unit is u (unified atomic mass unit) or Da (Dalton), although AMU may still be used. The AMU is used to express the relative masses of, and thereby differentiate between, various isotopes of elements.
1 u = 1 Da = 1 amu (in modern usage) = 1 g/mol
Hydrogen, for example, is the first element on the periodic table and has an atomic number of 1 and an atomic mass of 1.00794 amu, or atomic mass units. One amu is equal to approximately 1.66 × 10 −24 grams.
Some Examples:
- A hydrogen-1 atom has a mass of 1.007 u (or Da or amu).
- A carbon-12 atom is defined as having a mass of 12 u.
- The largest known protein, titin, has a mass of 3 x 106 Da.