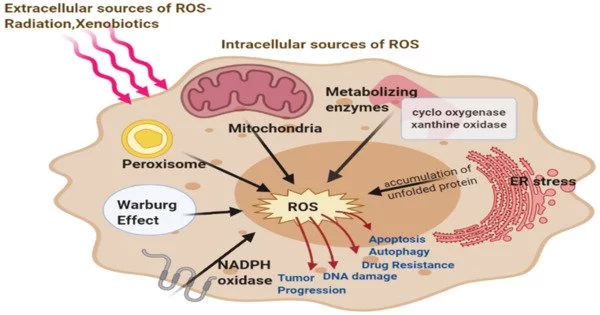
Antioxidants are substances that help protect cells from free radical damage, which is caused by unstable molecules that can harm cells and DNA. A recent study from Karolinska Institutet published in The Journal of Clinical Investigation demonstrates that vitamin C and other antioxidants induce the creation of new blood vessels in lung cancer tumors. The discovery supports the notion that antioxidant-rich dietary supplements can hasten tumor growth and spread.
“We discovered that antioxidants activate a mechanism that causes cancer tumors to form new blood vessels, which is surprising because antioxidants were previously thought to have a protective effect,” says study leader Martin Bergö, professor at the Department of Biosciences and Nutrition and vice president of Sweden’s Karolinska Institutet. “The new blood vessels nourish the tumours and can help them grow and spread.”
Antioxidants, which neutralize free oxygen radicals that might harm the body, are typically found in nutritional supplements. However, very high amounts can be dangerous. “There’s no need to be afraid of antioxidants in normal food, but most people don’t need extra amounts,” Professor Bergö explains. “In fact, it can be harmful to cancer patients and people at high risk of cancer.”
Our study opens the door to more effective ways of preventing angiogenesis in tumours; for example, patients whose tumours exhibit high levels of BACH1 might benefit more from anti-angiogensis therapy than patients with low BACH1 levels.
Ting Wang
Previously unknown mechanism
Professor Bergö’s group previously demonstrated that antioxidants such as vitamin C and E increase the growth and spread of lung cancer by stabilizing a protein known as BACH1. BACH1 is activated when the number of free oxygen radicals decreases, which can occur when more antioxidants are added to the diet or when spontaneous changes in tumor cells activate endogenous antioxidants. The researchers have now demonstrated that activating BACH1 causes the development of new blood vessels (angiogenesis).
While low oxygen levels (hypoxia) are known to be essential for angiogenesis in cancer tumors, the novel mechanism discovered by the researchers shows that tumors can generate new blood vessels even in the presence of normal oxygen levels. The research also demonstrates that BACH1 is regulated in a manner comparable to the HIF-1 protein, a process that was given the 2019 Nobel Prize in Physiology or Medicine and allows cells to adapt to fluctuations in oxygen levels. According to the latest study, HIF-1 and BACH1 collaborate in tumors.
Hoping for more effective drugs
“Many clinical trials have evaluated the efficacy of angiogenesis inhibitors, but the results have not been as successful as anticipated,” says Ting Wang, doctoral student in Professor Bergö’s group at Karolinska Institutet. “Our study opens the door to more effective ways of preventing angiogenesis in tumours; for example, patients whose tumours exhibit high levels of BACH1 might benefit more from anti-angiogensis therapy than patients with low BACH1 levels.”
The researchers used a variety of cell-biological methodologies and focused on lung cancer tumors by investigating organoids, which are miniature cultured microtumours from patients. They did, however, study mice as well as human breast and kidney tumor tissues. Tumors that were triggered by BACH1, either through ingested antioxidants or by overexpression of the BACH1 gene, developed more new blood vessels and were particularly susceptible to angiogenesis inhibitors.
“The next step is to investigate in depth how oxygen and free radical levels can regulate the BACH1 protein, and we will continue to assess the clinical relevance of our findings,” adds Ting Wang. “We’ll also conduct similar research in other cancer types, such as breast, kidney, and skin cancer.”