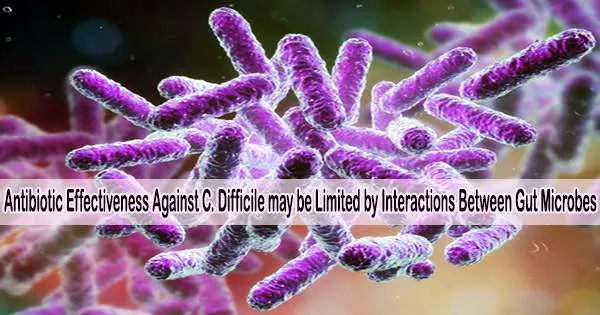
The bacteria Clostridioides difficile (C. difficile) can cause life-threatening colon damage in addition to diarrhea when it infects the large intestine. C. difficile infections typically occur when the normal balance of bacteria in the colon is disrupted, often due to the use of antibiotics.
A study published May 11, 2023, in PLOS Biology by Ophelia Venturelli at University of Wisconsin-Madison, U.S., and colleagues suggests that between-species interactions within the gut microbiome may impact the efficacy of antibiotics aimed at treating C. difficile infections.
Gut bacteria, also known as gut microbiota or gut microbiome, refers to the community of microorganisms that live in our digestive tract, primarily the large intestine. These microorganisms consist of bacteria, archaea, viruses, fungi, and other microbes.
Infections with C. difficile happen in the context of intricate resident gut ecosystems. Antibiotic regimens, however, are created without taking into account the interactions between C. difficile and other bacteria and instead rely on the bacterium’s measured susceptibility to medicines in monoculture.
Researchers tested the efficacy of two antibiotics used to treat C. difficile infections, vancomycin and metronidazole, in a broad human gut community in order to better understand susceptibility-altering microbial interactions across various microbial populations.
The human gut pathogen Clostridioides difficile (C. difficile) is embedded in a dense and diverse human gut community that influences its colonization ability, growth and functions. Using a bottom-up community assembly approach, we demonstrate that C. difficile’s response to clinically relevant antibiotics can be altered by an antibiotic-induced reduction in the strength of bacterial competition or global shifts in C. difficile’s cellular state in the presence of the commensal gut species Desulfovibrio piger.
Ophelia Venturelli
They developed a computational model to comprehend the impact of microbial interactions and drugs on C. difficile growth in order to find the ecological principles behind the interspecies interactions.
Researchers discovered that C. difficile may thrive in the absence of ecological competition and multiplies in abundance in the presence of sub-lethal quantities of the antibiotic metronidazole. These bacteria are more susceptible to the tested antibiotics than C. difficile.
The authors also discovered that C. difficile is resistant to metronidazole when a particular bacterial species is present. Future research should concentrate on how interspecies interactions may affect the efficacy of other therapeutically relevant medications as the study was only able to examine the effects of two antibiotics.
According to the authors, “These results provide key insights into ecological principles and molecular mechanisms influencing antibiotic susceptibility in this health-relevant system. Our work demonstrates that pathogen growth can be altered by inter-species interactions across a wide range of antibiotic concentrations, which should be considered in the design of antibiotic treatments.”
Venturelli adds, “The human gut pathogen Clostridioides difficile (C. difficile) is embedded in a dense and diverse human gut community that influences its colonization ability, growth and functions. Using a bottom-up community assembly approach, we demonstrate that C. difficile’s response to clinically relevant antibiotics can be altered by an antibiotic-induced reduction in the strength of bacterial competition or global shifts in C. difficile’s cellular state in the presence of the commensal gut species Desulfovibrio piger.”