What is the maximum weight of an element? An international team of researchers discovered that ancient stars could produce elements with atomic masses greater than 260, which are heavier than any element found naturally on Earth. The discovery adds to our understanding of element formation in stars.
We are literally made of star material. Stars are element factories, where elements constantly fuse or break apart to create other elements that are lighter or heavier. When we talk about light or heavy elements, we’re referring to their atomic mass. In general, atomic mass is determined by the number of protons and neutrons in the nucleus of one atom of a given element.
Only in neutron stars are the heaviest elements known to be created via the rapid neutron capture process, or r-process. Consider a single atomic nucleus floating in a neutron soup. Suddenly, a large number of those neutrons become attached to the nucleus in a very short period of time – usually less than one second – and then undergo some internal neutron-to-proton changes, and voila! A heavy element like gold, platinum, or uranium is formed.
The most massive elements are unstable or radioactive, which means they decay over time. They do this in part by splitting, a process known as fission.
The r-process is necessary if you want to make elements that are heavier than, say, lead and bismuth. You have to add many neutrons very quickly, but the catch is that you need a lot of energy and a lot of neutrons to do so.
Ian Roederer
“The r-process is necessary if you want to make elements that are heavier than, say, lead and bismuth,” says Ian Roederer, associate professor of physics at North Carolina State University and lead author of the research. Roederer was previously at the University of Michigan.
“You have to add many neutrons very quickly, but the catch is that you need a lot of energy and a lot of neutrons to do so,” Roederer says. “And the best place to find both are at the birth or death of a neutron star, or when neutron stars collide and produce the raw ingredients for the process.
“We have a general idea of how the r-process works, but the conditions of the process are quite extreme,” Roederer says. “We don’t have a good sense of how many different kinds of sites in the universe can generate the r-process, we don’t know how the r-process ends, and we can’t answer questions like, how many neutrons can you add? Or, how heavy can an element be? So we decided to look at elements that could be made by fission in some well-studied old stars to see if we could start to answer some of these questions.”
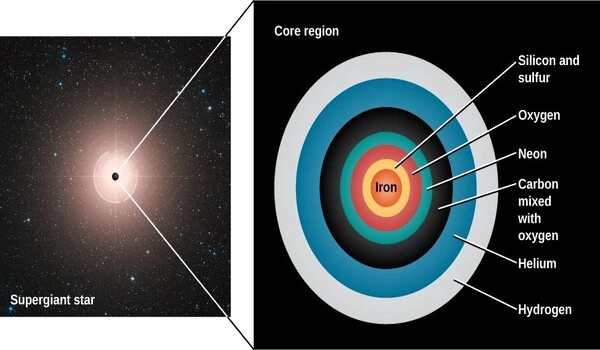
Ancient stars aided in the formation of heavy elements in the universe. Nucleosynthesis, or the creation of elements heavier than helium, occurs in the cores of stars via nuclear fusion reactions.
The researchers examined the amounts of heavy elements in 42 well-studied Milky Way stars for the first time. Heavy elements formed by the r-process were known to exist in earlier generations of stars. They discovered previously unknown patterns by taking a broader view of the amounts of each heavy element found in these stars collectively, rather than individually, as is more common.
These patterns indicated that elements near the middle of the periodic table, such as silver and rhodium, were most likely the byproducts of heavy element fission. The researchers discovered that the r-process can generate atoms with an atomic mass of at least 260 before they fission.
“That 260 is interesting because we haven’t previously detected anything that heavy in space or naturally on Earth, even in nuclear weapon tests,” Roederer goes on to say. “But seeing them in space gives us guidance for how to think about models and fission — and could give us insight into how the rich diversity of elements came to be.”