Six cellular layers make up the human cerebral cortex, but at Precision Neuroscience, a group of researchers and engineers is attempting to create a machine that resembles a seventh layer.
The Layer 7 Cortical Interface is a brain implant that intends to enable paralyzed patients to use just neural signals to control digital equipment. This implies that people suffering from severe degenerative illnesses like ALS will once again be able to connect with loved ones by mentally moving cursors, typing, and even accessing social media.
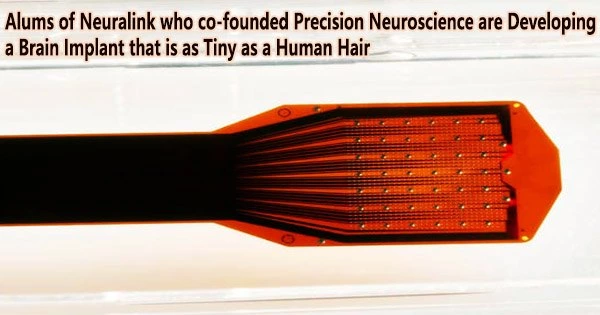
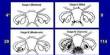
The Layer 7 is an electrode array that is thinner than a human hair and resembles a piece of scotch tape. This allows it to adhere to the surface of the brain without causing any tissue damage.
One of several businesses in the developing brain-computer interface, or BCI, sector is Precision, launched in 2021. Several businesses have successfully developed devices with this capability known as BCIs, which are systems that read brain impulses and transform them into commands for external technology.
Precision was co-founded by Benjamin Rapoport, who also co-founded Elon Musk’s BCI company, Neuralink, and Michael Mager. But while Neuralink’s BCI is designed to be implanted directly into the brain tissue, Precision relies on a surgical technique that is designed to be less invasive.
A surgeon who makes a very small incision in the skull and inserts the device in like a letter into a letterbox implants the Layer 7 array. Mager, who is also Precision’s CEO, said the slit is less than a millimeter thick so small that patients do not even need their hair shaved for the procedure.
“I think that’s a big advantage compared to technologies that require, for example, a craniotomy, removing a significant portion of the skull, which takes a lot of time and has a lot of risk of infection,” he told CNBC. “I’ve never met anyone who wanted a hole drilled into their skull.”
I think that the brain is, in a lot of ways, the next frontier for modern medicine. The fact that there are so many people who have neurological impairments of one sort or another, and that we have such crude tools to offer them, is going to change. It is changing.
Michael Mager
The nature of the procedure allows Precision to easily scale up the number of electrodes on the array, which Mager said will eventually allow the device to be used for neurological applications beyond paralysis.
If patients change their minds and decide they do not want the implant or prefer newer models in the future, the operation can be reversed.
“As you start thinking about rolling this out to larger patient populations, the risk-reward of any procedure is a fundamental consideration for anyone considering medical technology,” Mager said. “If your system is either irreversible, or potentially damaging upon explantation, it just means the commitment that you’re making to getting the implant is that much greater.”
Jacob Robinson, associate professor of electrical engineering at Rice University and founder of the BCI company Motif Neurotech, said Precision is making exciting strides in the minimally invasive BCI space. He said that it’s not just patients who have to weigh the risks and benefits of a procedure, but physicians and insurance companies as well.
Robinson said physicians have to weigh procedures quantitatively and based on existing literature, while insurance companies have to weigh the costs for their patients, so the less invasive surgery makes it easier on all three parties.
“It’s lower risk, but it also means that there’s an opportunity to treat more people, there’s greater adoption,” he said.
But because the device isn’t inserted directly into the brain tissue, Robinson said the resolution of the brain signals is not going to be as strong as it is in some other BCI devices.
“You get much better resolution than you would from outside the skull, not quite as high resolution as you go into the tissue,” he said. “But there’s a lot that you can do with this kind of medium scale.”
Precision has successfully used its Layer 7 device to decode neural signals in animals, and Mager said he hopes to get FDA approval to test the technology in humans in coming months.
The company announced a $41 million Series B funding round Wednesday, bringing its total to $53 million in under two years. The funding will allow Precision to hone its product, hire more employees and accelerate toward FDA regulatory review, a goal Mager said Precision is working toward quickly.
“We don’t want the next 15 years to be like the last 15 years, where this helps a few dozen people. So I think we’re in a hurry,” he said. “What we hear consistently from patients is, ‘We want this, and we want it sooner rather than later.’”
Mager said he thinks this year is proving to be a “watershed year” in neurotechnology, and that there has been a lot of positive momentum in the BCI space in terms of funding.
Though he said he understands the skepticism that exists around BCIs and technology as a whole, Mager said he thinks there is a real potential to make a difference for millions of people suffering from neurological conditions.
“I think that the brain is, in a lot of ways, the next frontier for modern medicine,” he said. “The fact that there are so many people who have neurological impairments of one sort or another, and that we have such crude tools to offer them, is going to change. It is changing.”