A chemical compound is aluminium sulfate; it is a popular aluminum salt, susceptible to hydrolysis, acid is an aqueous solution. Its chemical formula Al2(SO4)3. It is soluble in water and is primarily used in the purification of drinking water and wastewater treatment plants as a coagulating agent (promoting particle collision by neutralizing the charge) and also in the manufacture of paper. In solution, this agent adsorbs and precipitates protein antigens; the resulting precipitate increases the immunogenicity of the vaccine by promoting the slow release of antigen from the vaccine depot established at the inoculation site.

Aluminium Sulfate (crystal)
In order to produce a new form of salt called potassium aluminum sulfate K2SO4 • Al2(SO4) 3 • 24H2O, this salt is called complex salt, also known as aluminum potassium alum, commonly known as alum. This salt is called complex salt, also known as aluminum potassium alum, commonly known as alum. The anhydrous type occurs naturally as an unusual millosevichite mineral, found, for example, in volcanic environments and in burning dumps of coal-mining waste. From the point of view of the composition, an adduct of two simple salts is formed, which is not a simple mixture of the two salts, but a compound of the same crystal structure, a certain composition. As an anhydrous salt, aluminum sulfate is rarely, if ever, found. It forms a variety of various hydrates, the most common of which are hexadecahydrate Al2(SO4)3•16H2O and octadecahydrate Al2(SO4)3•18H2O.
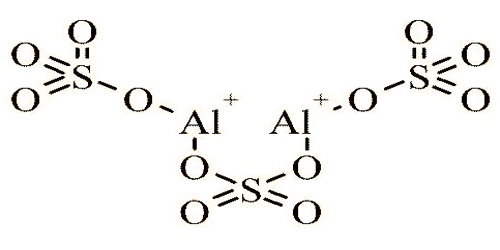
Liquid chemical structure of Aluminium Sulfate
A white crystalline solid is anhydrous aluminum sulfate; it is also generated as an Al2(SO4)3.18H2O 18-hydrate. In some sectors, it is also called the alum or papermaker’s alum. However, with the generic formula XAl(SO4)2•12H2O, where X is a monovalent cation such as potassium or ammonium, the term ‘alum’ is more generally and correctly used for any double sulfate salt. The distinction between complex salts and complex salts is that complex salts present simple ions without complexions in the solid-state or solution. The hazard to the atmosphere is the primary risk. To restrict its spread to the community, immediate measures should be taken.
It is possible to create aluminium sulfate by adding aluminium hydroxide, Al(OH)3, to sulfuric acid, H2SO4:
2 Al(OH)3 + 3 H2SO4 → Al2(SO4)3 + 6H2O
or by heating aluminum metal in a sulfuric acid solution:
2 Al + 3 H2SO4 → Al2(SO4)3 + 3 H2↑

Uses of Aluminium Sulfate
Aluminium sulfate is used in the manufacture of paper, in foams for firefighting, and in the treatment of sewage and water purification. It is also used as a firming agent in the human food industry, where it takes on the E number E520, and as a bactericide in animal feed. Using plastic bags lined with plastic glass bags for wrapping, aluminum sulfate readily absorbs water for agglomeration. Storage and transport can avoid humidity and rain. It is a typical adjuvant of the vaccine and works ‘by facilitating the slow release of antigen from the vaccine depot established at the inoculation site.’
Information Sources: