The bacteria, fungi, viruses, and protozoa found in our digestive tracts and on our skin, known as the microbial biome, or microbiome, may play a significant role in determining our health, well-being, moods, longevity, and even weight. According to microbiome researchers, the microbiome may be as important as DNA in shaping our individual destinies.
UT Southwestern researchers used artificial intelligence to discover a new family of sensing genes in enteric bacteria that are linked by structure and likely function but not genetic sequence. The findings, published in PNAS, provide a new method for determining the role of genes in unrelated species and may lead to new approaches to fighting intestinal bacterial infections.
“We identified similarities in these proteins in reverse of how it’s usually done. Instead of using sequence, Lisa looked for matches in their structure,” said Kim Orth, Ph.D., Professor of Molecular Biology and Biochemistry, who co-led the study with Lisa Kinch, Ph.D., a bioinformatics specialist in the Department of Molecular Biology.
We identified similarities in these proteins in reverse of how it’s usually done. Instead of using sequence, Lisa looked for matches in their structure. We had never seen anything like VtrC. But, we thought, other proteins like it must exist.
Kim Orth
Dr. Orth’s lab has long been interested in the mechanisms by which bacteria in marine and estuarine environments cause infections. Dr. Orth and her colleagues used biophysics in 2016 to characterize the structure of two proteins called VtrA and VtrC complex, which work together in the bacterial species Vibrio parahaemolyticus. She and her colleagues then discovered that the VtrA/VtrC complex in V. parahaemolyticus, which is frequently the cause of food poisoning from contaminated shellfish, detects bile on the bacterial cell surface and sends a signal to initiate a chemical cascade that causes this microbe to invade the intestinal cells of its human host.
Although VtrA shares some structural features with a protein called ToxR found in a related bacteria called Vibrio cholerae that causes cholera, it was unclear whether a homolog for VtrC also existed in this or any other bacteria.
“We had never seen anything like VtrC,” said Dr. Kinch. “But, we thought, other proteins like it must exist.”
The task has been reduced to minutes with the help of artificial intelligence (AI) and the most advanced computing hardware, identifying at a glance every microbe in a person’s biome, detecting patterns and changes invisible to the human eye, and analyzing interactions.
The study of microbial communities found in and on the human body is known as human microbiome analysis. The goal of human microbiome profiling research is to better understand how microbes affect health and disease. NGS has enabled studies capable of surveying the genomes of entire microbial communities, including those of unculturable organisms.
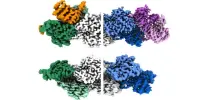
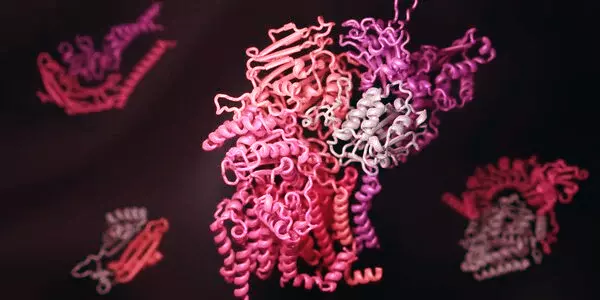
Without any known genes with sequence identities similar to VtrC, the researchers turned to software released just two years ago called AlphaFold. This artificial intelligence program can accurately predict the structure of some proteins based on the genetic sequence that codes for them – information that previously was only gleaned through laborious work in the laboratory.
AlphaFold demonstrated that a protein called ToxS in Vibrio cholerae has a very similar structure to VtrC, despite the fact that the two proteins share no recognizable genetic sequences. When the researchers looked for proteins with similar structural features in other organisms, they discovered homologs for VtrC in a number of other enteric bacteria species that cause human disease, including Yersinia pestis (which causes bubonic plague) and Burkholderia pseudomallei (which causes a tropical infection called melioidosis). Each of these VtrC homologs appears to work in tandem with proteins that are structurally similar to VtrA, implying that their roles in V. parahaemolyticus are the same.
Dr. Orth said these structural similarities could eventually lead to pharmaceuticals that treat conditions caused by different infectious organisms that rely on similar pathogenic strategies.