Activation energy
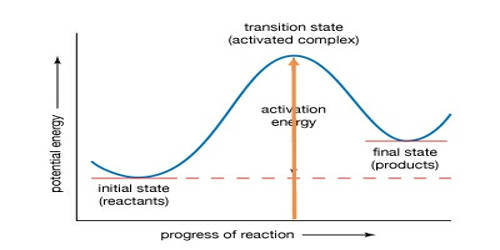
The minimum energy which must be available to a chemical system with potential reactants in order to result in a chemical reaction. Activation energy is needed so reactants can move together, overcome forces of repulsion, and start breaking bonds. To understand activation energy, we must first think about how a chemical reaction occurs.
In chemistry and physics, Energy of Activation is the energy that must be provided to a chemical or nuclear system with potential reactants to result in: a chemical reaction, nuclear reaction, or various other physical phenomena. The source of activation energy is typically heating, with reactant molecules absorbing thermal energy from their surroundings. For example, activation energy is needed to start a car engine. Turning the key causes a spark that activates the burning of gasoline in the engine. The combustion of gas won’t occur without the spark of energy to begin the reaction.
The activation energy of a chemical reaction is closely related to its rate. The activation energy (Ea) of a reaction is measured in joules (J), kilojoules per mole (kJ/mol) or kilocalories per mole (kcal/mol). The higher the barrier is, the fewer molecules that will have enough energy to make it over at any given moment. The released energy helps other fuel molecules get over the energy barrier as well, leading to a chain reaction.
Activation energy can be thought of as the magnitude of the potential barrier (sometimes called the energy barrier) separating the minima of the potential energy surface pertaining to the initial and final thermodynamic state. Once a reactant molecule absorbs enough energy to reach the transition state, it can proceed through the remainder of the reaction. For a chemical reaction, or division to proceed at a reasonable rate, the temperature of the system should be high enough such that there exists an appreciable number of molecules with translational energy equal to or greater than the activation energy. Many reactions have such high activation energies that they basically don’t proceed at all without an input of energy. For instance, the combustion of a fuel like propane releases energy, but the rate of reaction is effectively zero at room temperature.
The term Activation Energy was introduced in 1889 by the Swedish scientist Svante Arrhenius. In transition-state theory, the activation energy is the difference in energy content between atoms or molecules in an activated. If chemical reactions did not have reliable activation energy requirements, we would live in a dangerous world.