About Radionuclide
Definition
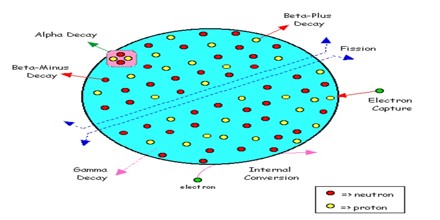
A Radionuclide (radioactive nuclide or radionuclides) is an unstable nuclide and thus degenerates emitting ionizing radiation. Although some physicists sometimes commonly used to designate the palabraradioisótopo however, it should be noted that strict or formal language of physics and nuclear technology is flawed as a nuclide and an isotope are not the same. Radioactive decay is a random process at the level of single atoms: it is impossible to predict when one particular atom will decay. However, for a collection of atoms of a single element the decay rate, and thus the half-life for that collection can be calculated from their measured decay constants. The range of the half-lives of radioactive atoms have no known limits and span a time range of over 55 orders of magnitude.
Radionuclides occur naturally and are artificially produced in nuclear reactors, cyclotrons, particle accelerators or radionuclide generators. There are about 730 radionuclides with half-lives longer than 60 minutes. With the longest half lives are the 32 primordial radionuclides that have survived from the creation of the Solar System. Over 60 further radionuclides are detectable in nature, either as daughters of these, or through natural production on Earth by cosmic radiation. More than 2400 radionuclides have half-lives less than 60 minutes. Most of those are only produced artificially, and have very short half-lives. For comparison, there are about 254 stable nuclides.
Radionuclides apply to nuclear technology for electricity in industry, medicine and nuclear weapons, primarily for the propulsion of vehicles and tools to kill. Its use implies serious environmental risks (radioactive contamination) and health (radiotoxicity, radiation poisoning, etc.), so it must be done with extreme care. Recall that naturally occurring radionuclides such as uranium or plutonium exist in finite quantities on Earth, so you have to use them in a sustainable manner. Moreover, their use generates radioactive waste, which can be very dangerous and for which the only treatment is usually done is cover until its radioactivity is close to the natural. Possible treatments for those who take more than thirty years to do all the spent fuel from nuclear power plants and for military purposes, for example are still in the process of theory, research or experimentation.
Uses of Radionuclide
A radionuclide is an atom with an unstable nucleus. It is characterized by excess energy that it radiates and emits. Radionuclide Radionuclide are used in two general ways:
For Chemical Properties: In which familiar atoms are used as tracers because they are very similar to non-radioactive nuclides.
As Source of Radiation: They are used as chemical tracers that emit certain kinds of radiation that are used for chemical purposes. Uses of Radionuclide a Radioactive Tracer is a chemical compound in which one or two atoms have been replaced by radioactive isotopes.
Its radioactivity helps it identify the mechanism of different chemical reactions by following the paths taken from just reactants, all through the reaction, and then when they come products.
Uses of Tracers in Imaging Imaging Imaging is a procedure used for Nuclear Medicine but is too broad to be contained under one topic, therefore it stands alone.
Nuclear Medicine is a medical specialty that uses radioactive substances to deal with diseases.
In biology, radionuclides of carbon can serve as radioactive tracers because they are chemically very similar to the nonradioactive nuclides, so most chemical, biological, and ecological processes treat them in a nearly identical way. One can then examine the result with a radiation detector, such as a Geiger counter, to determine where the provided atoms were incorporated.
Agriculture Radionuclides are used to stop the sprouting of root crops after harvesting. They are also used to kill parasites and pests, by emitting gamma-rays on the crops. The radionuclide are also used to emit gamma-rays that control the ripening of different fruit and vegetables.
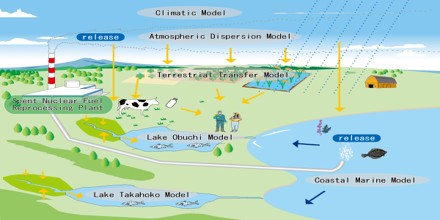
Environment Radionuclides are used to trace and analyze pollutants, to study the movement of surface water, and to measure water runoffs from rain and snow. Natural radionuclides are used in geology, archaeology, and paleontology to measure ages of rocks, minerals, and fossil materials.
Industry In industry, and in mining, radionuclides: examine welds, to detect leaks, to study the rate of wear, erosion and corrosion of metals, and for on-stream analysis of a wide range of minerals and fuels.
Impacts on Organisms
Radionuclides that find their way into the environment may cause harmful effects as radioactive contamination. They can also cause damage if they are excessively used during treatment or in other ways exposed to living beings, by radiation poisoning. Potential health damage from exposure to radionuclides depends on a number of factors, and “can damage the functions of healthy tissue/organs. Radiation exposure can produce effects ranging from skin redness and hair loss, to radiation burns and acute radiation syndrome. Prolonged exposure can lead to cells being damaged and in turn lead to cancer. Signs of cancerous cells might not show up until years, or even decades, after exposure.