About Proton
Definition
Proton is a stable subatomic particle in the baryon family having a mass of 1.672 × 10-24 grams (1,836 times that of the electron) and a positive electric charge of approximately 1.602 × 10-19 coulombs. Proton is a tiny particle, smaller than an atom. The number of protons in the atomic nucleus is that which determines the atomic number of an element, as indicated in the periodic table of the elements.
Protons and neutrons, each with masses of approximately one atomic mass unit, are collectively referred to as “nucleons”. One or more protons are present in the nucleus of every atom; they are a necessary part of the nucleus. The number of protons in the nucleus is the defining property of an element, and is referred to as the atomic number.
This number gives each element its unique identity. In the atoms of any particular element, the number of protons in the nuclei is always the same. An atom of simple hydrogen has a nucleus consisting of a single proton all by itself. The nuclei of all other elements nearly always contain neutrons in addition to protons.
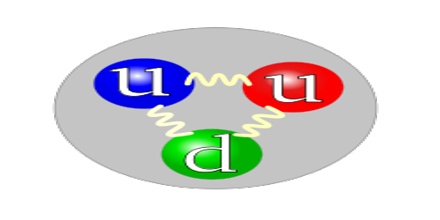
Proton is classified as a baryon, and is composed of three quarks (uud). The corresponding antiparticle, the antiproton, has the same characteristics as the proton but with negative electric charge. It is stable by itself. In some rare types of radioactive decay emit free protons, and the result of the decomposition of free neutrons in other disintegrations. As a free proton, it has the facility to pick up an electron and become a neutral hydrogen isotope, which can react chemically very easily. Free protons may exist in plasmas, cosmic rays or in the solar wind.
Stability of Proton
The proton is a baryon and is considered to be composed of two up quarks and one down quark. One of the implications of the grand unification theories is that the proton should decay with a half-life on the order of 1032 years. The nature of quark confinement suggests that the quarks are surrounded by a cloud of gluons, and within the tiny volume of the proton other quark-antiquark pairs can be produced and then annihilated without changing the net external appearance of the proton.
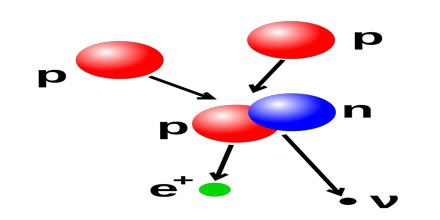
A free proton is a stable particle that has not been observed to break down spontaneously to other particles. Free protons are found naturally in a number of situations in which energies or temperatures are high enough to separate them from electrons, for which they have some affinity. Free protons are emitted directly from atomic nuclei in some rare types of radioactive decay. Protons also result along with electrons and antineutrinos, from the radioactive decay of free neutrons, which are unstable.
Relations and Functions of Protons and Neutrons
Protons and neutrons are nucleons. Both are linked in the nucleus by a strong nuclear force. The most common hydrogen isotope is a nucleus with a proton. The nuclei of the heavy hydrogen isotopes (deuterium and tritium) contain one proton and one or two neutrons, respectively. These two hydrogen isotopes are used as nuclear fuel in nuclear fusion reactions. All other types of atoms are composed of two or more protons and a different number of neutrons.
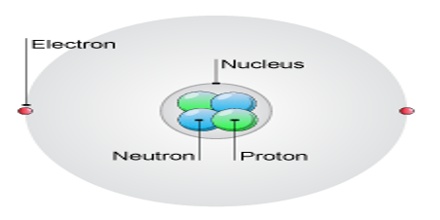
Protons need not be confined to the nuclei of atoms. When protons are found outside atomic nuclei, they acquire fascinating, bizarre, and potentially dangerous properties, similar to those of neutrons in similar circumstances. They carry an electric charge; they can be accelerated by electric and/or magnetic fields. High-speed protons, and atomic nuclei containing them, are emitted in large numbers during solar flares.
The number of protons in the nucleus of an atom determines its chemical properties and, therefore, the chemical element is represented by the number of protons in a nucleus (Z). To determine the isotopes of an element, the number of neutrons (N) is also used by summing all the nucleons, and is known as the mass number (A).
Reference: techtarget.com, nuclear-energy.net, dictionary.com, wikipedia.