Actin filaments are dynamic structures that play important roles in a variety of cellular processes such as cell movement, shape determination, and intracellular transport. These filaments are made up of actin monomers that polymerize into long helical chains. Actin filaments are naturally polar, with “plus” and “minus” ends. The positive end is where new actin monomers are added, while the negative end is where monomers are lost.
An electron microscopy study revealed important details about actin filaments, which are structural components of cells and muscles.
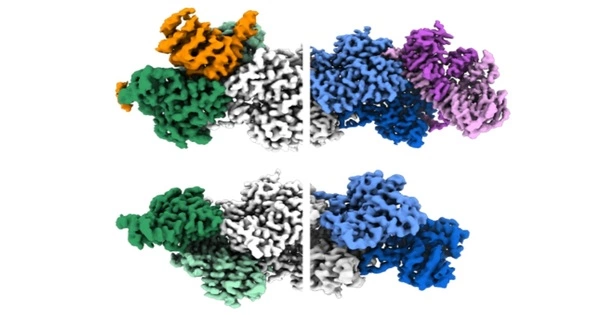
Actin filaments, which are protein structures that are essential for living movement from single cells to animals, have long been known to have polarity associated with their physical properties, with growing “barbed” and shrinking “pointed” ends. The filament ends also interact with other proteins in cells in different ways. However, scientists have never fully understood the mechanism underlying these differences. Researchers from the University of Pennsylvania’s Perelman School of Medicine have now revealed key atomic structures of the ends of the actin filament using cryo-electron microscopy (cryo-EM).
The study, published in Science, provides fundamental insights that may help fill in the details behind some muscle, bone, heart, neurological, and immune disorders caused by actin defects or deficiencies.
The results of our study provide a mechanistic understanding of a process we have known about for more than 40 years, referred to as filament treadmilling, and impacts how we view the cellular roles of actin in health and disease.
Roberto Dominguez
Actin is the most abundant protein found inside the cells of higher organisms like animals. It serves as the foundation for filaments, which are long, thin structures that provide structural support as part of the cell’s “cytoskeleton,” the system that gives cells shape and polarity. Actin filaments undergo rapid changes, which underpin key cellular events such as movement along surfaces, cell-to-cell contact, and cell division. Actin filaments are also important components of muscle fibers.
“The results of our study provide a mechanistic understanding of a process we have known about for more than 40 years, referred to as filament treadmilling, and impacts how we view the cellular roles of actin in health and disease,” said the study senior author Roberto Dominguez, Ph.D., the William Maul Measey Presidential Professor of Physiology at Penn.
The dynamics of actin filaments are largely governed by the “treadmilling” process, in which individual actin proteins are shed from one filament end, known as the pointed end, and added at the other, barbed end. Actin filaments can be stabilized by specific “capping” proteins that bind to the filament ends and prevent further addition or loss of individual actin proteins. Many other proteins bind to the barbed and pointed ends of the actin filament. However, the structural details determining the specificity of these interactions – the details that explain why these two ends function so differently – have been murky.
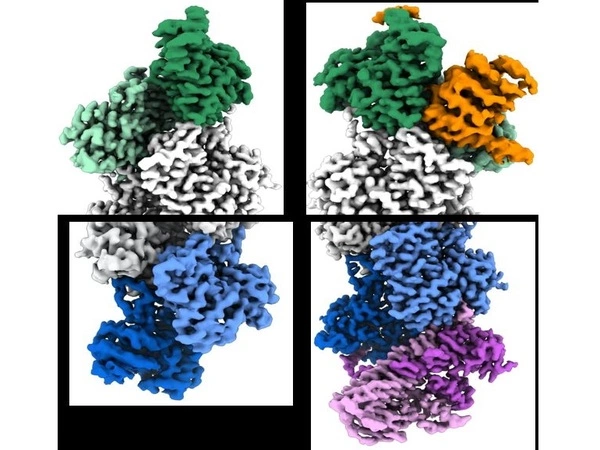
The researchers used cryo-EM to examine actin filaments in their study, which included two Penn students, Peter Carman, Ph.D., a recent graduate student in Dominguez’s lab, and Kyle Barrie, Ph.D., a graduate student currently in the lab, who served as co-first authors. A researcher uses this high-resolution imaging technique to capture thousands of snapshots of a target molecule, align them computationally, and then average them to reduce random image “noise” – resulting in a 3-D reconstruction of the molecule that may be sharp enough to see individual atoms.
With artificial intelligence (AI) assistance, the researchers were able to focus on the ends of the filaments instead of their middle, as had previously been the norm in similar research. By doing so, they identified hundreds of thousands of filament end views, allowing them to obtain near-atomic scale reconstructions. These revealed a “flat” actin shape, or conformation, at the uncapped barbed end, versus a “twisted” conformation at the uncapped pointed end.
The findings also included information on the structural changes caused by two actin filament-capping proteins, CapZ at the barbed end and tropomodulin at the pointed end. These are the two proteins found at the ends of the filament in skeletal and cardiac muscles, and they play an important role in the stabilization of actin filaments in muscle fibers. Our muscles would fall apart if these proteins were not present.
The findings of this study provide critical mechanistic details for a better understanding of actin biology in general. These findings, according to the researchers, should aid in understanding and, ultimately, treatment of disorders caused by actin dysfunction.