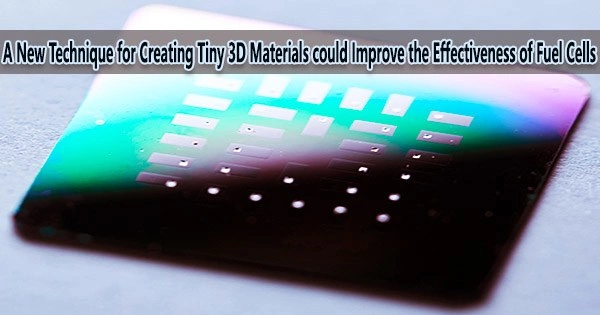
Researchers from UNSW Sydney have developed a cutting-edge method for fabricating microscopic 3D materials that may ultimately make fuel cells and hydrogen batteries more affordable and environmentally friendly.
In a paper published in Science Advances, scientists from UNSW Science’s School of Chemistry demonstrate how interconnected hierarchical structures may be successively “grown” in 3D at the nanoscale and endowed with special chemical and physical properties that facilitate energy conversion reactions.
In chemistry, hierarchical structures are arrangements of elements such as molecules within a structure of other elements, which may or may not be ordered. In the natural world, such as in flower petals and tree branches, we can observe similar phenomena. The level at the nanoscale, however, where these structures have great promise, is invisible to the human sight.
Using conventional methods, scientists have found it challenging to replicate these 3D structures with metal components on the nanoscale. There are 10 millimeters in one centimeter, so you can see how tiny these 3D materials have to be. In only one of those millimeters, there are one million minuscule segments, each of which is one nanometre, or nm.
“To date, scientists have been able to assemble hierarchical-type structures on the micrometer or molecular scale,” says Professor Richard Tilley, Director of the Electron Microscope Unit at UNSW and senior author of the study. “But to get the level of precision needed to assemble on the nanoscale, we needed to develop an entirely new bottom-up methodology.”
The researchers created complicated chemical molecules out of simpler ones using a technique called chemical synthesis. They were able to produce 3D hierarchical structures with diameters of roughly 10–20 nanometers by carefully growing nickel branches with hexagonal crystal structures on cores with cubic crystal structures.
These new 3D nanostructures are engineered to expose more atoms to the reaction environment, which can facilitate more efficient and effective catalysis for energy conversion. If this is used in a fuel cell or battery, having a higher surface area for the catalyst means the reaction will be more efficient when converting hydrogen into electricity.
Professor Richard Tilley
Due to the direct connection of a metallic core and branches, the resulting interconnected 3D nanostructure has a high surface area, excellent conductivity, and surfaces that may be chemically changed.
Due to these characteristics, it is a perfect candidate for use as an electrocatalyst, which is a chemical that increases the rate of reactions in the oxygen evolution reaction, a critical step in the energy conversion process. The nanostructure’s properties were examined using electrochemical analysis from state-of-the-art electron microscopes provided by the Electron Microscope Unit.
“Growing the material step by step is a contrast to what we do assembling structures at the micrometer level, which is starting with bulk material and etching it down,” says the lead author of the study Dr. Lucy Gloag, a Postdoctoral Fellow at the School of Chemistry, UNSW Science. “This new method allows us to have excellent control over the conditions, which lets us keep all of the components ultra-small on the nanoscale where the unique catalytic properties exist.”
Nanocatalysts in fuel cells
The majority of the atoms in conventional catalysts, which are frequently spherical, are trapped in the center of the sphere. The majority of the material is squandered since it cannot participate in the reaction environment because there are so few atoms on the surface.
“These new 3D nanostructures are engineered to expose more atoms to the reaction environment, which can facilitate more efficient and effective catalysis for energy conversion,” Prof. Tilley says.
“If this is used in a fuel cell or battery, having a higher surface area for the catalyst means the reaction will be more efficient when converting hydrogen into electricity,” Prof. Tilley says.
Dr. Gloag says it means that less of the material needs to be used for the reaction.
“It will eventually decrease the costs as well, making energy production more sustainable and ultimately shifting our dependence further away from fossil fuels.”
The next phase of the research will focus on modifying the material’s surface using platinum, a superior catalytic metal but one that is more expensive. About a sixth of the cost of an electric car alone is the platinum powering the fuel cell.
“These exceptionally high surface areas would support a material like platinum to be layered on in individual atoms, so we have the absolute best use of these expensive metals in a reaction environment,” Prof. Tilley says.