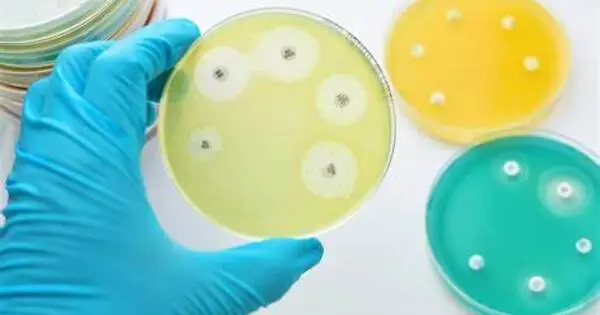
Mount Sinai’s Icahn School of Medicine researchers have discovered a novel way to bacterial infection control. The findings were published online in Nature Structural & Molecular Biology.
The researchers discovered a strategy to activate a critical bacterial defense mechanism in order to combat and manage bacterial illnesses. The cyclic oligonucleotide-based antiphase signaling system (CBASS) is a natural mechanism utilized by some bacteria to defend themselves against viral infections. Bacteria self-destruct to prevent the virus from spreading to other bacterial cells in the population.
“We wanted to see how the bacterial self-killing CBASS system is activated and whether it can be leveraged to limit bacterial infections,” says co-senior author Aneel Aggarwal, PhD, Professor of Pharmacological Sciences at Icahn Mount Sinai. “This is a fresh approach to tackling bacterial infections, a significant concern in hospitals and other settings. It’s essential to find new tools for fighting antibiotic resistance. In the war against superbugs, we need to constantly innovate and expand our toolkit to stay ahead of evolving drug resistance.”
We wanted to see how the bacterial self-killing CBASS system is activated and whether it can be leveraged to limit bacterial infections. This is a fresh approach to tackling bacterial infections, a significant concern in hospitals and other settings.
Aneel Aggarwal
According to a 2019 Centers for Disease Control and Prevention report, more than 2.8 million antimicrobial-resistant infections occur in the United States each year, resulting in over 35,000 deaths.
The researchers investigated how “Cap5,” or CBASS-associated protein 5, is activated for DNA degradation and how it can be used to regulate bacterial infections using structural analysis as well as numerous biophysical, biochemical, and cellular studies. Cap5 is a crucial protein that, when triggered by cyclic nucleotides (small signaling molecules), destroys the bacterial cell’s DNA.
“In our study, we started by identifying which of the many cyclic nucleotides could activate the effector Cap5 of the CBASS system,” says co-senior author Olga Rechkoblit, PhD, Assistant Professor of Pharmacological Sciences at Icahn Mount Sinai. “Once we figured that out, we looked closely at the structure of Cap5 when it’s bound to these small signaling molecules. Then, with expert help from Daniela Sciaky, Ph.D., a researcher at Icahn Mount Sinai, we showed that by adding these special molecules to the bacteria’s environment, these molecules could potentially be used to eliminate the bacteria.”
The researchers discovered that establishing the structure of Cap5 with cyclic nucleotides was a technical hurdle that required expert assistance from Dale F. Kreitler, PhD, AMX Beamline Scientist at Brookhaven National Laboratory. It was accomplished utilizing micro-focused synchrotron X-ray radiation from the same facility. Micro-focused synchrotron X-ray radiation is a type of X-ray radiation that is both produced by a certain type of particle accelerator (synchrotron) and precisely concentrated or focused on a small region for more comprehensive imaging or analysis.
The researchers will then investigate how their findings relate to different types of bacteria and determine whether their technology may be used to treat infections caused by a variety of hazardous bacteria.