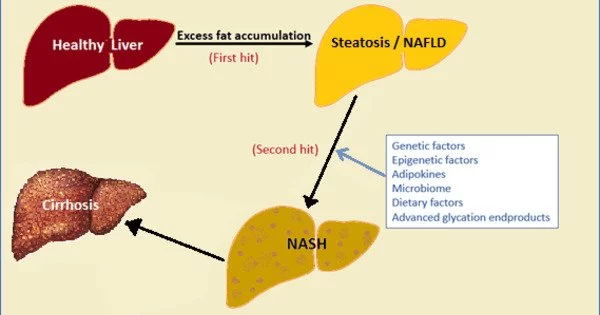
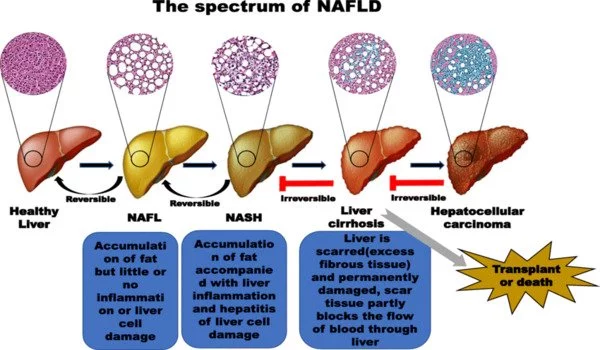
Non-alcoholic Fatty Liver Disease (NAFLD) refers to a group of conditions caused by an excess of fat in the liver in people who consume little or no alcohol. Fat should be absent or minimal in healthy liver cells. More than 5% fat stored in liver cells is considered excessive and can lead to fatty liver. NAFLD is thought to affect one in every three people in the UK.
A study led by Texas A&M AgriLife Research provides compelling evidence of the critical role of hepatocyte adenosine kinase in the progression of NAFLD. The study, titled “Hepatocyte Adenosine Kinase Promotes Excessive Fat Deposition and Liver Inflammation,” was published in the scientific journal Gastroenterology in September.
Hepatocytes are cells in the liver that play critical roles in metabolism, detoxification, protein synthesis, and innate immunity. An important player in the proper function of hepatocytes is an enzyme called adenosine kinase, ADK. However, the current study shows that ADK can also drive liver disease progression.
The research demonstrated that hepatocyte adenosine kinase promotes excessive fat deposition and liver inflammation and is a key factor in the pathogenesis of nonalcoholic fatty liver disease. There is already some evidence as to how making dietary changes can be effective in helping prevent or reduce the severity of NAFLD.
Chaodong Wu
“We aimed to study whether hepatocyte ADK functions to promote excessive fat deposition and liver inflammation,” said Chaodong Wu, M.D., Ph.D., an AgriLife Research Faculty Fellow in the Texas A&M Department of Nutrition. Wu is also a Presidential Impact Fellow of Texas A&M University and the corresponding author for the study.
Wu said NAFLD is highly associated with obesity and progresses to an advanced stage when the liver develops overt inflammatory damage. This study pointed to the idea that hepatocyte ADK may be an important therapeutic target for managing obesity and NAFLD.
“The research demonstrated that hepatocyte adenosine kinase promotes excessive fat deposition and liver inflammation and is a key factor in the pathogenesis of nonalcoholic fatty liver disease,” he said. “There is already some evidence as to how making dietary changes can be effective in helping prevent or reduce the severity of NAFLD. If further research validates the effects of dietary components on inhibiting adenosine kinase in hepatocytes, scientists could develop new approaches for managing this disease.
About the study
The researchers examined how changes in ADK activity relate to levels of liver inflammation and amount of fat in the liver. The team studied mice that were genetically engineered to have either more or less ADK activity than normal.
The team fed the mice a high-fat diet for 12 weeks or a methionine-choline deficient diet, which is known to contribute to liver injury, for five weeks. Under these diets, mice with lowered ADK activity in their hepatocytes had lower levels of fat and inflammation in the liver than controls. In contrast, mice with elevated ADK activity had greater body weight and increased body fat, liver fat and liver inflammation levels than normal mice.
Going further, the team conducted experiments to clarify exactly how ADK activity causes these effects.
Wu said the study included the analysis of cell-to-cell crosstalk using single-cell RNA sequencing, which was done with assistance from James Cai, Ph.D., a researcher in Texas A&M’s Department of Veterinary Integrative Biosciences.
“The study’s analysis of lipid profiles in mouse liver samples indicated increased presence of lipids that promote mitochondrial dysfunction, leading in turn to liver inflammation,” Wu said.
The team went on to analyze ADK activity and liver health in human liver samples. Like in mice, the data showed that decreased levels of ADK correlated with lower levels of NAFLD, while increased levels of the enzyme were linked to exacerbated liver steatosis and inflammation. The results also showed that ADK promotes excessive fat deposition and liver inflammation through suppressing fatty acid oxidation in liver cells.
“The amount of hepatocyte ADK protein was positively correlated with the degrees of hepatic steatosis in livers from human subjects,” Wu said. “This novel finding highlights the enzyme’s pathological importance in human NAFLD.”