Scientists have made progress in developing a new type of painkiller that targets a specific receptor in the body. This new painkiller is designed to reduce the risk of addiction and overdose compared to traditional opioid painkillers. However, this is still an ongoing area of research and it will take some time before a new painkiller is available on the market.
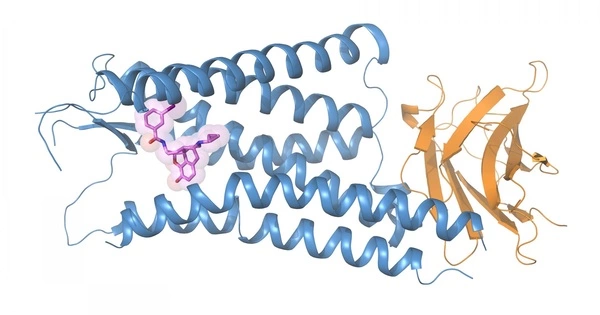
The new study offers a comprehensive structural framework that should aid drug developers in rationally designing safer drugs to relieve severe pain. In an ongoing effort to improve opioid pain relievers, American and Chinese researchers used cryoEM technology to solve the detailed structures of the entire opioid receptor family bound to their naturally occurring peptides. Following that, structure-guided biochemical studies were carried out to better understand the mechanisms of peptide-receptor selectivity and signaling drugs.
This work, published in Cell, provides a comprehensive structural framework that should help drug developers rationally design safer drugs to relieve severe pain. This work was spearheaded by the lab of Eric Xu, PhD, at the CAS Key Lab of Receptor Research in China, in collaboration with the lab of Bryan L. Roth, MD, PhD, at the UNC School of Medicine, where graduate student Jeff DiBerto led the pharmacological experiments to understand the receptors’ signaling mechanisms.
The problem in the field has been a lack of molecular understanding of the interplay between opioid peptides and their receptors. This understanding was required in order to rationally design potent and safe peptide or peptide-inspired drugs.
Michael Hooker
Opioid pain relievers work by mimicking a naturally occurring pain-relief function in our nervous system. They are the most powerful pain relievers we have. Unfortunately, they have severe side effects such as numbness, addiction, and respiratory depression, which lead to overdose deaths.
For many years, scientists have tried in various ways, all involving one or more of the four opioid receptors, to overcome the side-effect problem. One avenue that scientists are continuing to investigate is the development of peptide or peptide-inspired small molecule drugs.
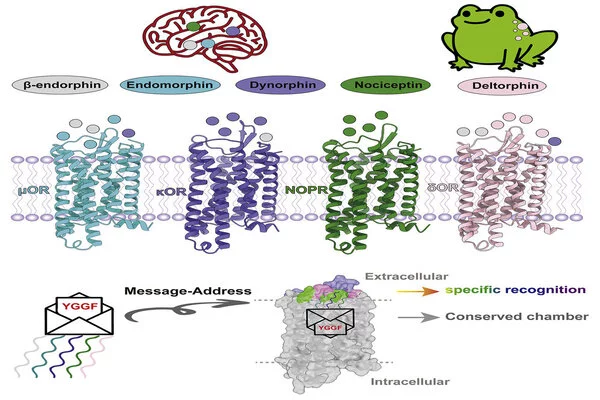
Peptides are short chains of amino acids; think of them as short proteins. Certain naturally occurring, or endogenous, peptides bind to opioid receptors on the surface of cells to create an analgesic effect, also known as pain relief. Think of an analgesic like an anesthetic, except that analgesics do not “turn off” the nerves to numb the body or alter consciousness. So, the idea is to create a peptide drug that has a strong analgesic effect, without numbing nerves or altering consciousness or causing digestive, respiratory, or addiction issues.
“The problem in the field has been a lack of molecular understanding of the interplay between opioid peptides and their receptors,” said Michael Hooker Distinguished Professor of Pharmacology and co-senior author Roth. “This understanding was required in order to rationally design potent and safe peptide or peptide-inspired drugs.”
The Xu and Roth labs solved the detailed structures of endogenous peptides bound to all four opioid receptors using cryogenic electron microscopy, or cryoEM, and a battery of biomechanistic experiments in cells. These structures revealed details and insights into how naturally occurring opioid peptides recognize and activate opioid receptors selectively. Exogenous peptides, or drug-like compounds, were also used in some of the experiments to learn how the receptors are activated.
The cryoEM structures of agonist-bound receptors in complex with their G protein effectors (referred to as their “active state”) represent how these receptors appear when they are signaling in cells, providing a detailed view of peptide-receptor interactions. The Roth lab used the Xu lab’s structures to guide the design of mutant receptors, which were then tested in biochemical assays in cells to see how they altered receptor signaling. Understanding these interactions can then be used to create drugs that are selective for opioid receptor subtypes, as well as to generate certain signaling outcomes that may be more beneficial than those produced by conventional opioids.
“This collaboration revealed conserved, or shared, mechanisms of activation and recognition of all four opioid receptors, as well as differences in peptide recognition that can be exploited for creating subtype-selective drugs,” said DiBerto, first author and PhD candidate in the Roth lab. “We provide more needed information to keep pushing the field forward, to answer basic science questions we hadn’t been able to answer before now.”
Previous research revealed the structure of opioid receptors in their inactive or active-like states, with active state structures only found in the mu-opioid receptor subtype, which is the primary target of drugs such as fentanyl and morphine. The authors of the Cell paper show agonist-bound receptors in complex with their G protein effectors, which was made possible by cryoEM technology that did not exist when currently used medications were developed.
Oxycontin, oxycodone, and morphine all have different effects inside cells and throughout the nervous system, including pain relief. However, they also have effects on the digestive and respiratory systems, and they interact with cells to cause addiction. Fentanyl, meanwhile, is another powerful pain reliever, but it binds to opioid receptors in such a way as to cause severe side effects, including the shutdown of the respiratory system.