Researchers at the University of Oxford have made a significant advancement in the development of tiny, bio-integrated devices that can stimulate cells directly. The work has been published today in the journal Nature.
Small bio-integrated devices having the ability to communicate with and excite cells may find use in a variety of therapeutic procedures, such as the rapid healing of wounds and the administration of targeted medication therapies. However, such devices all need a power source to operate. To date, there has been no efficient means to provide power at the microscale level.
To combat this, scientists from the University of Oxford’s Department of Chemistry have created a tiny power source that can modify the behavior of human nerve cells in culture. The apparatus employs internal ion gradients to produce energy, drawing inspiration from how electric eels do it.
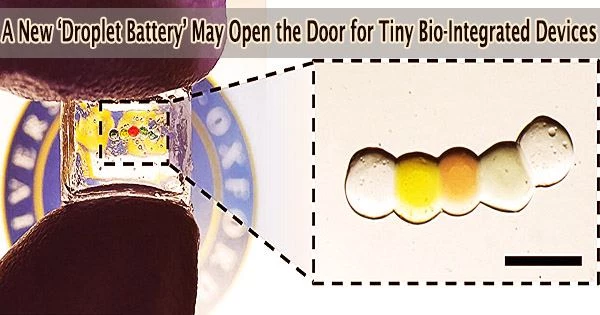
The miniaturized soft power source is produced by depositing a chain of five nanolitre-sized droplets of a conductive hydrogel (a 3D network of polymer chains containing a large quantity of absorbed water).
Because each droplet is unique, a gradient in salt content is produced along the chain. Lipid bilayers that give mechanical support while obstructing ion movement between the droplets keep the droplets apart from one another.
The power source is turned on by cooling the structure to 4°C and changing the surrounding medium: this disrupts the lipid bilayers and causes the droplets to form a continuous hydrogel. This enables the ions to pass from the high-salt droplets at the two ends to the low-salt droplet in the center of the conductive hydrogel.
The miniaturized soft power source represents a breakthrough in bio-integrated devices. By harnessing ion gradients, we have developed a miniature, biocompatible system for regulating cells and tissues on the microscale, which opens up a wide range of potential applications in biology and medicine.
Dr. Yujia Zhang
The energy generated from the ion gradients is converted into electricity by attaching the end droplets to electrodes, allowing the hydrogel structure to function as a power source for external components.
The triggered droplet power source in the study generated a current that lasted for more than 30 minutes. The maximum output power of a unit made of 50 nanolitre droplets was around 65 nanowatts (nW). The devices produced a similar amount of current after being stored for 36 hours.
The study team then showed how real cells could be linked to one end of the apparatus so that the ionic current could control their activity directly. The device was linked to droplets of human neural progenitor cells that were fluorescently labelled to show that they were active.
When the power source was turned on, time-lapse recording demonstrated waves of intercellular calcium signalling* in the neurons, induced by the local ionic current.
Dr. Yujia Zhang (Department of Chemistry, University of Oxford), the lead researcher for the study, said: “The miniaturized soft power source represents a breakthrough in bio-integrated devices. By harnessing ion gradients, we have developed a miniature, biocompatible system for regulating cells and tissues on the microscale, which opens up a wide range of potential applications in biology and medicine.”
The device’s modular construction, according to the researchers, would enable many units to be combined to increase the voltage and/or current generated. This may make it possible to power upcoming wearable technology, bio-hybrid interfaces, implants, artificial tissues, and microrobots.
By combining 20 five-droplet units in series, they were able to illuminate a light-emitting diode, which requires about 2 Volts. They believe that by automating the manufacturing process, such as by employing a droplet printer, they could create droplet networks made up of tens of thousands of power units.
Professor Hagan Bayley (Department of Chemistry, University of Oxford), the research group leader for the study, said: “This work addresses the important question of how stimulation produced by soft, biocompatible devices can be coupled with living cells. The potential impact on devices including bio-hybrid interfaces, implants, and microrobots is substantial.”
Neurons communicate with one another in order to coordinate biological processes like gene transcription, synaptic plasticity, neuronal firing, and neurotransmitter release, such as calcium signaling.