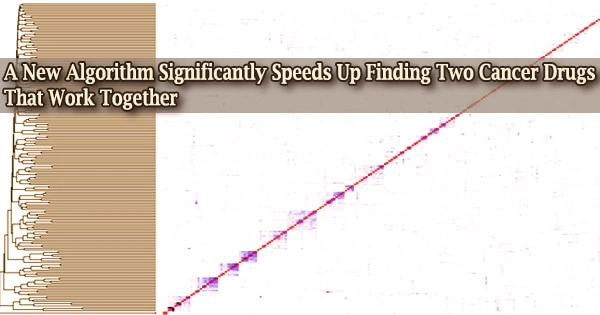
Bioinformatics experts have devised an algorithm that can shorten the time it takes to find two or more pharmaceuticals that work together to treat a condition like cancer or viral infection from years to months.
According to Dr. Richard McIndoe, director of the Center for Biotechnology and Genomic Medicine at the Medical College of Georgia, the new algorithm enables researchers to use extensive existing databases with information about how one cancer drug changed the gene expression of a specific breast cancer cell line and how well it killed the cell, then mathematically combine those results with the impact of another drug to see if they could work better together.
The algorithm does speed up the way to the trials, he claims, even though it does not immediately make the kind of information available that would initiate a clinical trial.
“The idea is we ultimately want to find these synergistic drug combinations that will hopefully help patients with cancer,” McIndoe says. “For researchers it becomes a particularly faster way to find those synergistic combinations, without having to screen one drug at a time, which is really not feasible.”
According to the researchers’ findings published in the journal PLOS ONE, medication combination therapies are increasingly being used to treat cancer because they can increase therapeutic efficacy, decrease drug dosage (and associated toxicity), and overcome drug resistance.
“It’s not uncommon for the cancer to become resistant to chemotherapy drugs so one of the ways that clinicians try to get around that is using combinations, two chemotherapy drugs together,” McIndoe says. “The likelihood that you will develop resistance to both of them simultaneously is lower than if you had just one.”
However, the researchers claim that there are no efficient, effective methods to determine the optimal medicine combinations given the abundance of drugs and pharmacological combinations on the market.
Additionally, not all pharmacological combinations are advantageous; in fact, one medication may serve as an antagonist against another, essentially preventing or at the very least weakening the therapeutic action of the latter. According to McIndoe, the appropriate combination will improve the treatment’s effectiveness, which implies that when used together, they are more effective at killing cancer cells.
It’s not uncommon for the cancer to become resistant to chemotherapy drugs so one of the ways that clinicians try to get around that is using combinations, two chemotherapy drugs together. The likelihood that you will develop resistance to both of them simultaneously is lower than if you had just one.
Dr. Richard McIndoe
The algorithm also fosters collaboration among researchers by making it simple to share results, which allows for the evaluation of additional medications and cell lines and the quick expansion of the database of beneficial drug combinations for fighting particular tumors.
“The tricky part is how do you determine which drug combinations have a synergistic effect,” he says.
There are currently large, automated stations where various drug combinations are put with a particular cancer cell line to examine what occurs in an effort to find the best combination. According to McIndoe, the list of medications is lengthy and the number of possible combinations is considerably greater.
According to him, another method involves combining medications based on what is known about each of their individual mechanisms of action. This method is both slow and expensive because it involves using a number of different drugs and drug combinations.
The Library of Integrated Network-based Cellular Signatures project is just one of the enormous databases of cell lines that have been treated with a single drug to examine the impact on gene expression, before and after treatment. These databases can be used to streamline large-scale studies like the ones the MCG investigators wanted to conduct.
They concentrated on 57 randomly chosen chemotherapy drugs from the database, carefully examining the molecular changes each drug produced and tying that to growth rate, or how many cancer cells each drug killed. They then created a mathematical model of the molecular changes and the amount of killing for each.
“Since we have all the single drug effects, we can mathematically combine two drugs based on their molecular changes,” McIndoe says.
In total, they looked at 1,596 different combinations of the 57 cancer medications. According to McIndoe and his colleagues, their algorithm selected 30 of the best drug combinations, eight of which were then verified using a widely used statistical model called ZIP.
This result was significantly better than chance and required much less money and time than testing the large number of potential drug combinations. Additional laboratory studies where the suggested synergistic combinations were used to treat cancer cell lines further supported their synergy.
He points out that it would take almost three years to screen all 1,596 options using conventional methods as opposed to only eight weeks using their technique.
“What we were asking for this paper is can we use that gene expression data to come up with a way to prioritize which drugs would have the highest probability of being synergistic when you put them together,” McIndoe says.
A significant factor in the emergence and spread of cancer is alterations in gene expression or mutations brought on by factors such as exposure to the environment or even unintentional errors. The DNA of cancer cells is damaged by several cancer treatment classes in various ways, which stops or at least slows the reproduction of cancer cells.
“When you hit a cell with anything, such as a drug or a nutrient change, the cell responds,” he says. “It responds in a way where it is going to start to change its gene expression profile.”
The alterations are probably the result of the cancer cell fighting to survive, such as trying to activate a different signaling channel to promote growth since the medication has shut down the established one. However, if the medication is effective, the cell will perish, therefore McIndoe and his associates utilized kill rate as their key performance indicator.
Only a few of the 57 medications were discovered to have no effect on the cancer cell line, according to the researchers, and those pharmaceuticals were not shown to have a synergistic effect. However, synergy is still conceivable when these treatments are coupled with the appropriate partner, according to McIndoe.
They argue that the algorithm might also be used to more easily choose the optimal treatment combinations for conditions like bacterial, fungal, and viral infections.
The following steps entail examining additional breast cancer cell lines that have been treated with those same 57 medicines, reexamining their molecular responses, and determining whether those responses are consistent across cell lines.
Additionally, McIndoe intends to develop a database where other researchers may quickly upload the effect on gene expression and growth rate for their studies and take the critical next step in laboratory animal experiments to see, for instance, whether the synergy persists in an intact tumor.
Dr. Jiaqi Li, a former graduate student at McIndoe who took on the project for his thesis to employ bioinformatics tools to advance science, is the study’s first author. At the MCG Center for Biotechnology and Genomic Medicine, Li is currently a research associate. Human population geneticist and biostatistician Dr. Hongyan Xu is a co-author and works at the MCG Department of Population Health Sciences.