Novel chemical compounds derived from a fungus could open up new avenues for treating colorectal cancer, one of the most frequent and deadly malignancies worldwide. Researchers in the journal Angewandte Chemie reported on the isolation and characterization of a hitherto unknown family of metabolites (terpene-nonadride heterodimers). One of these chemicals successfully kills colorectal cancer cells by targeting the enzyme DCTPP1, making it a potential biomarker and therapeutic target.
Rather than employing traditional cytostatic medications, which have several adverse effects, current cancer treatment typically comprises targeted tumor therapies aimed at specific target molecules in tumour cells. However, the prognosis for colorectal cancer patients remains bleak, highlighting the need for new targets and newer medicines.
Most targeted tumor medicines are based on tiny compounds found in plants, fungus, bacteria, and marine species. Approximately half of contemporary cancer treatments are derived from natural sources. A team led by Ninghua Tan, Yi Ma, and Zhe Wang from China Pharmaceutical University (Nanjing, China) picked Bipolaris victoriae S27, a fungus that lives on plants, as the beginning point for their quest for new medications.
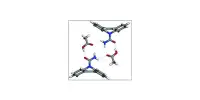
The team first analyzed metabolic products by cultivating the fungus under many different conditions. They discovered twelve unusual chemical structures belonging to a previously unknown class of compounds: terpene-nonadride heterodimers, molecules made from one terpene and one nonadride unit.
Ninghua Tan, Yi Ma, and Zhe Wang
The team first analyzed metabolic products by cultivating the fungus under many different conditions (OSMAC method, one strain, many compounds). They discovered twelve unusual chemical structures belonging to a previously unknown class of compounds: terpene-nonadride heterodimers, molecules made from one terpene and one nonadride unit.
Widely found in nature, terpenes are a large group of compounds with very varied carbon frameworks based on isoprene units. Nonadrides are nine-membered carbon rings with maleic anhydride groups. The monomers making up this class of dimers termed “bipoterprides” were also identified and were found to contain additional structural novelties (bicyclic 5/6-nonadrides with carbon rearrangements).

Nine of the bipoterprides were active against colorectal cancer cells. The most effective was bipoterpride No. 2, which destroyed tumor cells as well as the traditional cytostatic medication Cisplatin. In mouse tests, it reduced tumor size without causing any hazardous side effects.
The researchers employed a range of ways to investigate the drug’s mechanism. Bipoterpride 2 inhibits the enzyme dCTP-pyrophosphatase 1 (DCTPP1), which regulates the cellular nucleotide pool. The heterodimer bonds substantially more firmly than the individual monomers.
DCTPP1 activity is enhanced in certain types of malignancies, boosting cancer cell invasion, migration, and proliferation while suppressing programmed cell death. It can also make cancer cells more resistant to treatment. Bipoterpride 2 inhibits this enzymatic activity and disrupts the — pathologically altered — amino acid metabolism in the tumor cells.