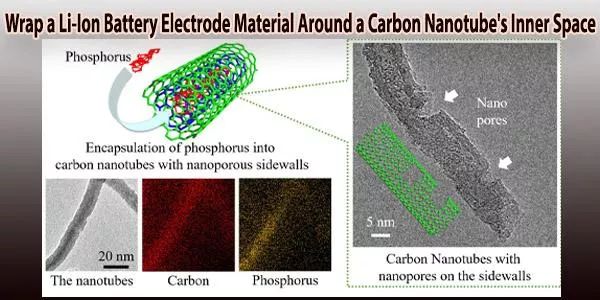
By introducing red phosphorus with a very high capacity into the inner space of carbon nanotubes (CNTs) with a tubular structure, researchers at the Toyohashi University of Technology have proven the electrochemical performance of lithium ion batteries (LIBs).
When accessible lithium ion channels, or nanopores, were created onto the sidewalls of the CNTs where the red phosphorus was enclosed, the electrodes showed an improvement in the electrochemical reactivity of the red phosphorus.
Additionally, reversible electrochemical processes and the comparatively high structural stability of red phosphorus in the nanotubes even after the fifty-first charge-discharge cycle were shown by the charge-discharge profiles and structural analyses.
When compared to the graphite utilized in commercial LIBs, the charge-discharge capacities are at least twice as great. As a result, a new electrode material with high capacity is suggested for LIBs.
Because it can theoretically give a capacity around seven times greater than that of graphite, which is employed as a commercial electrode material for LIBs, red phosphorus has drawn interest as a higher capacitive electrode material. It is believed that the significant difference in capacity is caused by an appropriate concentration of lithium ions in the structures of either graphite for LiC6 or phosphorus for Li3P.
However, throughout the lithium ion insertion and extraction processes, red phosphorus experiences significant volumetric changes, pulverization, and peeling off, which causes rapid capacity fading because there is less electrochemically reactive red phosphorus present.
We have proposed phosphorus-encapsulated CNTs as an electrode material for LIBs with high capacity, even though additional improvements in the structures are required to achieve long-term cycling without capacity fading.
Red phosphorus also suffers from a disadvantage in terms of energy loss during the insertion and extraction of lithium ions due to its low electrical conductivity.
Tomohiro Tojo and his colleagues at the Department of Electrical and Electronic Information Engineering, Toyohashi University of Technology, have synthesized unique structures in which red phosphorus is encapsulated into the inner spacing of CNTs to prevent its peeling off from the electrode and improve its electronic conductivity.
For improving the electrochemical reactivity of red phosphorus through accessible pathways of lithium ions, nanopores (<5 nm) were also formed onto the sidewalls of the phosphorus-encapsulated CNTs. The distribution of phosphorus atoms inside the nanotubes following phosphorus encapsulation demonstrated the structural stability of red phosphorus.
At the fifty-first charge-discharge cycle, a reversible capacity using CNT electrodes with phosphorus encapsulation revealed about 850 mAh/g. This was a number that was significantly greater than graphite electrodes’ value.
After the tenth cycle and subsequent cycles, the calculated ratio of charge to discharge capacities (Coulombic efficiencies) was >99%, which denotes a high degree of reversibility of charge-discharge processes on red phosphorus.
However, due to the breakdown of some P-P bonds and other side reactions on the surface of phosphorus and the CNTs, the charge-discharge capacities rapidly reduced with increasing cycle number.
Contrary to popular belief, the phosphorus-encapsulated CNT with nanopores enabled the electrochemical performance to significantly enhance over the phosphorus-encapsulated CNT without nanopores. The strong reactivity of red phosphorus with lithium ions through the sidewall nanopores is hypothesized to be the cause of this.
After the charge-discharge cycles, red phosphorus was observed to be inside the nanotubes.
We have proposed phosphorus-encapsulated CNTs as an electrode material for LIBs with high capacity, even though additional improvements in the structures are required to achieve long-term cycling without capacity fading. Further studies will be performed on the utilization of such electrodes.