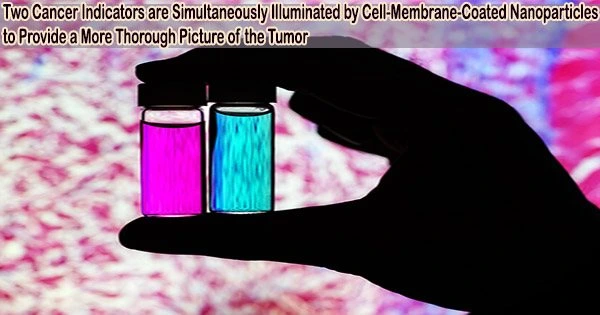
According to University of Illinois Urbana-Champaign researchers, new imaging agents that may illuminate numerous indicators simultaneously may soon allow cancer surgeons to get a more thorough view of tumors during surgery.
The red blood cell membrane-encased fluorescent nanoparticles target tumors more effectively than currently used clinically approved dyes. They also have the ability to emit two distinct signals in response to a single surgical light beam, a property that may make it easier for medical professionals to distinguish tumor borders and spot metastatic cancers.
The team’s paper, “Cell-membrane coated nanoparticles for tumor delineation and qualitative estimation of cancer biomarkers at single wavelength excitation in murine and phantom models,” is published in ACS Nano.
“The imaging agents can be combined with bioinspired cameras, which the researchers previously developed for real-time diagnosis during surgery,” said research group leader Viktor Gruev, an Illinois professor of electrical and computer engineering.
In the latest study, scientists used live mice and 3D tumor phantoms, which imitate the characteristics of actual tumors and their environment, to demonstrate their unique dual-signal nanoparticles.
This is appealing for surgical application, as it could help determine where exactly to make the cut. Having multiple signals gives a more overall picture of the tumor. And it could tell a surgeon, ‘This may be metastatic, you may want to be more aggressive in your removal.’
Indrajit Srivastava
“If you want to find all the cancer, imaging one biomarker is not enough. It could miss some tumors. If you introduce a second or a third biomarker, the likelihood of removing all cancer cells increases, and the likelihood of a better outcome for the patients increases,” said Gruev, who also is a professor in the Carle Illinois College of Medicine.
“Multiple-targeted drugs and imaging agents are a recent trend, and our group is driving the trend hard because we have the camera technology that can image multiple signals at once.”
“Traditionally, a surgeon removes a tumor and sends it to a pathologist for assessment, a process that can take hours to days,” said Illinois postdoctoral researcher Indrajit Srivastava, the first author of the paper.
As research has moved toward real-time diagnostics, several challenges have prevented wide application: Many tumor-targeted imaging agents only minimally reach their tumor targets, instead being quickly cleared from the bloodstream and accumulating in the liver, Srivastava said.
“A few people before us have used nanoparticles coated with red blood cells and found they circulate longer a few days. We saw the same thing in our mice: The membrane-coated nanoparticles circulated longer in blood, with reduced uptake in the liver. Because they were circulating longer, more of the imaging agents accumulated in the tumors, giving us a stronger fluorescent signal,” Srivastava said.
The two biomarkers that the new imaging agents are targeting are one that is common in early cancer and one that is common in late-stage cancer, which is more likely to spread to other organs. The scientists discovered that the probes were successful at separating the two signals as well as malignant tissue from healthy tissue.
“This is appealing for surgical application, as it could help determine where exactly to make the cut. Having multiple signals gives a more overall picture of the tumor. And it could tell a surgeon, ‘This may be metastatic, you may want to be more aggressive in your removal.’” Srivastava said.
“Only needing one wavelength of laser light to elicit multiple signals is another benefit for surgical applications, as it makes the instrumentation much more compact than those requiring multiple lasers for each needed wavelength,” Gruev said.
The researchers intend to continue preclinical and clinical research utilizing their dual-signal dyes with the surgical goggles they have created, as well as to create more tumor-imaging agents that target multiple markers.
“In this battle for ensuring we remove all the cancer cells during surgery, we need investments both in the imaging camera technology and in the tumor targeting agents,” Gruev said. “This work is helping us better realize and guide the holistic approach that we are taking as we are getting closer and closer to clinical trials.”