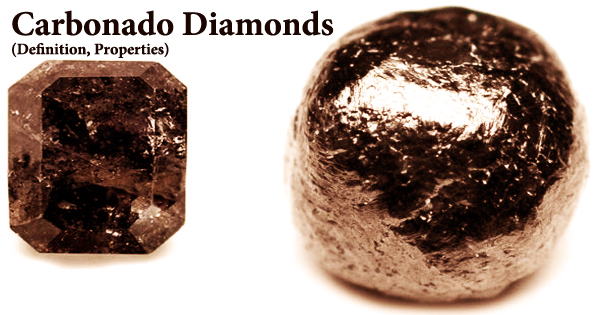
Troilite is a pyrrhotite mineral, which means it’s a rare iron sulfide mineral. Although it may be found on Earth, meteorites have a higher concentration of it. Pyrrhotite is an iron-deficient mineral with the formula Fe(1-x)S (x = 0 to 0.2). Troilite is non-magnetic because it lacks the iron deficit that gives pyrrhotite its distinctive magnetism. When a meteorite fell in Albareto, Italy in 1766, Domenico Troili gathered and analyzed samples of the mineral.
Gustav Rose, a German mineralogist, examined the sample later in 1862 and identified it as stoichiometric iron sulfide. Domenico Troili, the first person to find it, was given the name. The Apollo, Viking, and Phobos space missions all verified the presence of troilite on the Moon and potentially on Mars. Troilite is non-magnetic because it is stoichiometric FeS with no vacancies in its atomic structure, whereas magnetic pyrrhotite is iron-deficient.
It has a gray-black stripe, a metallic sheen, and high magnetic characteristics. It is opaque and non-fluorescent. Because the relative strengths of sulfur isotopes in meteorites are relatively stable compared to Earth minerals, troilite from the Canyon Diablo meteorite has been chosen as the worldwide sulfur isotope ratio standard. At flat surfaces, its cracks are irregular. It can be found in massive, tabular, or platy forms. The average density of troilite is 4.61 g/cm3, and its relative hardness varies from 3.5 to 4.

Troilite has a hexagonal shape, and its unit cell is made up of two vertically stacked basic NiAs-type pyrrhotite cells, with the top cell diagonally displaced. Though it may be considered a polytype of pyrrhotite in certain ways, IMA CNMNC considers it a grandfathered species. Troilite is also known as pyrrhotite-2C and may be found in serpentine (Del Norte County, California, USA), a layered ultramafic intrusive containing Fe–Cu–Ni sulfides (Sally Malay deposit, Australia), and as nodules in meteorites.
Silicates, phosphates, graphite, sphalerite, chromite, daubréelite, pyrite, chalcopyrite, valleriite, cubanite, mackinawite, pentlandite, and pyrrhotite are all found in close proximity. At T 122°C, troilite may convert into hexagonal pyrrhotite (pyrrhotite-2H polytype). It’s found in copper, nickel, platinum, and iron ore deposits in South Africa and Australia, where it’s found alongside pyrrhotite, pentlandite, mackinawite, cubanite, valleriite, chalcopyrite, and pyrite.
Troilite is a mineral that may be found in serpentine, layered ultramafic intrusives, and meteorites. Troilite is a rare mineral that may be found on Earth. It is very rare in the Earth’s crust (even pyrrhotite is uncommon compared to pyrite and other Iron(II) sulfate minerals). The majority of troilite on the planet comes from meteorites. It’s found in a lot of meteorites (including Lunar and Martian ones) and planetary nebulae. Canyon Diablo is the most well-known troilite-bearing meteorite.
Troilite seems to represent a significant phase in the mineral composition of the model rock of Jupiter’s Galilean satellites Ganymede and Callisto, according to measurements made by the Voyager mission in 1979 and Galileo in 1996. Because the sulfur isotopic ratio in meteorites is constant, but the sulfur isotopic composition of Earth materials changes owing to bacterial activity, the meteoritic standard was chosen. Troilite is expected to be a prevalent mineral in Martian surface rocks, based on Viking-1 and Viking-2 XRF soil studies and Phobos-2 orbital-ray data.
At the lunar surface, troilite is the most prevalent sulfide mineral. It makes up around 1% of the lunar crust and may be found in any rock or meteorite that came from the moon. Troilite is found in roughly 1% of all basalts brought back by the Apollo 11, 12, 15, and 16 missions. It’s frequently discovered in Martian meteorites (i.e. those originating from Mars). Troilite is found in a small percentage of Martian meteorites, similar to the Moon’s surface and meteorites.
Information Sources: