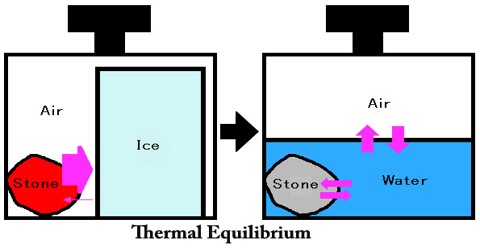
Thermal Equilibrium
Definition
Thermal Equilibrium is when two objects are at the same temperature. Two objects at different temperatures will reach thermal equilibrium because heat energy will flow from high temperature to low temperature. Heat energy flows from high temperature to low. In the summer heat goes from outside where it is 95 to inside. In the winter heat flows from the warm house to the outside. It is possible to force heat to flow the wrong way. This is done by refrigerators and air conditioners, but it requires energy. Work has to be done to make the heat go from cold to hot. People like to compare this to rolling a ball uphill. The ball won’t go uphill by itself, but anyone can make it go uphill by doing work. Air conditioners and heat pumps make heat go “uphill”.
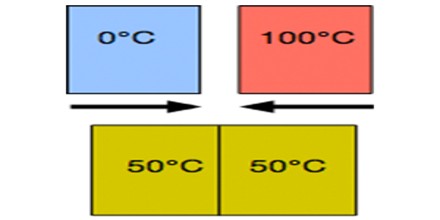
Thermal Equilibrium obeys the zeroth law of thermodynamics. A system is said to be in thermal equilibrium with itself if the temperature within the system is spatially and temporally uniform. The “zeroth law” states that if two systems are at the same time in thermal equilibrium with a third system, they are in thermal equilibrium with each other.
Mechanism and Formula of Thermal Equilibrium
Temperature is the average kinetic, or movement, energy of the molecules in a substance. When anyone put two objects of different temperatures in contact with one another, the faster-moving molecules in one material will collide with the slower-moving molecules in the other. The heat energy will gradually spread out until the two objects have the same temperature – until they have reached thermal equilibrium. This is basically the same as the second law of thermodynamics, which states that heat only spontaneously moves from hotter places to colder places, never the other way around.
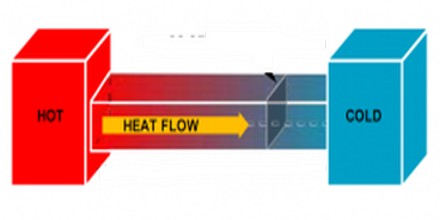
If an object (or system) is in thermodynamic equilibrium, then it can be said that the system has minimized its thermodynamic potential. There are many types of thermodynamic potential quoted in physics, but perhaps the most common one is the Helmholtz free energy, which measures the total amount of useful work that could be extracted from the system.
Types of Thermal Equilibrium
Relation of Thermal Equilibrium between Two Thermally Connected Bodies –
The relation of thermal equilibrium is an instance of contact equilibrium between two bodies, which means that it refers to transfer through a selectively permeable partition, the contact path. For the relation of thermal equilibrium, the contact path is permeable only to heat; it does not permit the passage of matter or work; it is called a diathermal connection. According to Lieb and Yngvason, the essential meaning of the relation of thermal equilibrium includes that it is reflexive and symmetric. It is not included in the essential meaning whether it is or is not transitive. After discussing the semantics of the definition, they postulate a substantial physical axiom, that they call the “zeroth law of thermodynamics”, that thermal equilibrium is a transitive relation. They comment that the equivalence classes of systems so established are called isotherms.
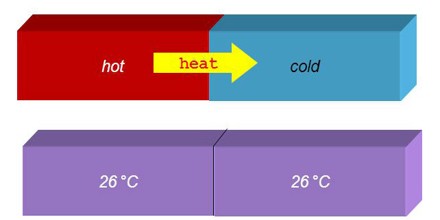
Internal Thermal Equilibrium of an Isolated Body –
Thermal Equilibrium of a body in itself refers to the body when it is isolated. The background is that no heat enters or leaves it, and that it is allowed unlimited time to settle under its own intrinsic characteristics. When it is completely settled, so that macroscopic change is no longer detectable, it is in its own thermal equilibrium. It is not implied that it is necessarily in other kinds of internal equilibrium.
Applications of Thermal Equilibrium

Refrigerator –
- When food is put in the refrigerator, the heat from the food is transferred into the air of the refrigerator.
- This process is continued until the temperature of the food equal to the temperature of the air in the refrigerator, when thermal equilibrium is reached between the food and the refrigerator.
Oven –
- When food such as meat or cake is put in the oven, the heat of the oven is transferred into the food.
- This process will continue until the food is in thermal equilibrium with the air in the oven.
- This happen when the temperature of the food is equal to the temperature of the air in the oven.
Thermometer –
- Thermometer is placed in contact with the patient’s body.
- If both the body temperature of the patient and that of the mercury (or alcohol) in the clinical thermometer have reached thermal equilibrium, then the temperature of the thermometer is the same as the body temperature, hence the reading of the thermometer shows the body temperature of the patient.