Catalyst surfaces provide atoms for chemical reactions. A metal surface, for example, adsorbs hydrogen atoms from a gas such as methane and feeds these atoms to an electrochemical reaction that generates electrical power in a hydrogen fuel cell. A catalyst is a substance that accelerates or reduces the temperature or pressure required to initiate a chemical reaction without being consumed in the process. Catalysis is the process of facilitating a reaction by adding a catalyst.
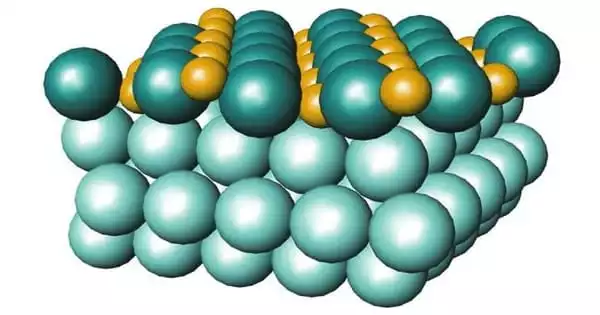
The three-dimensional structure of the surface of catalyst nanoparticles has been seen at atomic resolution by a research team. This structure is critical to the particles’ activity and stability. The precise insights were obtained using a combination of atom probe tomography, spectroscopy, and electron microscopy. Nanoparticle catalysts, for example, can be utilized to produce hydrogen for the chemical sector. To improve the performance of future catalysts, it is critical to understand how the three-dimensional structure affects it.
Researchers from Ruhr-Universität Bochum, the University of Duisburg-Essen, and the Max Planck Institute for Chemical Energy Conversion in Mülheim a der Ruhr collaborated on the study as part of the Collaborative Research Centre “Heterogeneous oxidation catalysis in the liquid phase.”
Atom probe tomography has huge potential to provide atomic insights into the compositional changes on the surface of catalyst nanoparticles during crucial catalytic events like oxygen evolution for hydrogen production or CO2 reduction.
Professor Tong Li
Nanoparticle catalysts are employed in the chemical sector to produce hydrogen. To improve the performance of future catalysts, it is critical to understand how the three-dimensional structure affects it. A German-Chinese research team has successfully observed the 3D structure of the surface of catalyst nanoparticles at atomic resolution in a new study. They used techniques such as atom probe tomography, spectroscopy, and electron microscopy.
A team led by Weikai Xiang and Professor Tong Li from Atomic-scale Characterisation collaborated with the Chairs of Electrochemistry and Nanoscale Materials and Industrial Chemistry at RUB. Institutes in Shanghai, China, and Didcot, UK, also participated. The team’s findings are published in the journal Nature Communications.
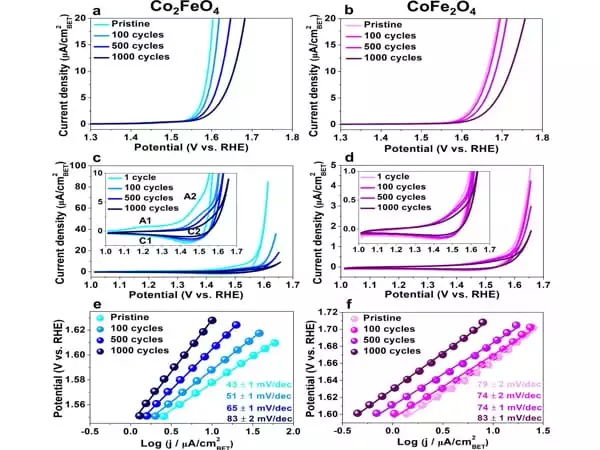
Particles observed during the catalysis process
The researchers looked at two types of nanoparticles made of cobalt iron oxide that were around ten nanometres in size. They examined the particles as they were catalyzed in the so-called oxygen evolution reaction. This is a half-reaction that occurs during water splitting for hydrogen production: hydrogen can be created by splitting water with electrical energy; in the process, hydrogen and oxygen are formed. The partial reaction in which oxygen is generated, i.e. the oxygen evolution reaction, is the bottleneck in the development of more efficient industrial methods. This reaction alters the catalyst surface, rendering it inactive over time. The surface structure and compositional alterations have a significant impact on the activity and stability of the electrocatalysts.
Obtaining precise information about what happens on the catalyst surface during the reaction remains a difficulty for small nanoparticles with a size of roughly ten nanometres. The researchers effectively visualized the distribution of different types of atoms in the cobalt iron oxide catalysts in three dimensions using atom probe tomography. They demonstrated how the structure and content of the surface changed during the catalysis process by combining it with other approaches – and how this change affected the catalytic performance.
The surface area of a solid will ultimately influence how fast a reaction between a solid and a liquid occurs. This is due to the fact that the liquid and solid can only collide at the liquid-solid contact, which is located on the solid’s surface. The solid molecules imprisoned within the solid’s body are unable to react. As a result, increasing the solid’s surface area exposes more solid molecules to the liquid, allowing for a faster reaction.
Catalysts are compounds that speed up reactions by lowering the activation energy required for the reaction to take place. Because a catalyst is not destroyed or altered during a reaction, it can be reused. For example, under normal circumstances, H2 and O2 do not combine. They do, however, mix in the presence of a small amount of platinum, which acts as a catalyst, and the reaction proceeds quickly.
“Atom probe tomography has huge potential to provide atomic insights into the compositional changes on the surface of catalyst nanoparticles during crucial catalytic events like oxygen evolution for hydrogen production or CO2 reduction,” Tong Li concludes.