Big data analysis can be a powerful tool in the fight against Alzheimer’s disease and other forms of dementia. By analyzing large datasets, researchers can identify patterns and trends that may not be apparent in smaller datasets. This can help them to better understand the causes of these diseases and to develop more effective treatments.
For example, researchers may use big data analysis to identify genetic risk factors for Alzheimer’s disease or to identify patterns in brain scans that are indicative of the disease. They may also use big data to identify potential new drugs or other therapies that could be effective in treating the disease.
In addition to helping researchers better understand Alzheimer’s disease, big data analysis can also be used to improve the diagnosis and treatment of patients with the disease. For example, doctors may use data from electronic health records and other sources to identify patients who are at high risk for developing Alzheimer’s and to tailor their treatment plans accordingly. Overall, big data analysis has the potential to significantly improve our understanding of Alzheimer’s disease and to develop more effective treatments for it.
Alzheimer’s disease has always had its puzzles and contradictions. For Pacific Northwest National Laboratory (PNNL) researcher Vladislav Petyuk, whose research on the progressive, age-related disease spans over a decade, some of the struggles have come from studies where “we can only connect the dots a pair at a time.”
Assessment of a patient’s brain and blood proteins, and other biological molecules, reveal patterns that can then be targeted for tailored intervention.
Petyuk
Petyuk’s research touches multiple areas in biological and computational science at PNNL. He has produced dozens of publications on Alzheimer’s disease. But now he sees the needle moving in the right direction.
“Over the last 10 years,” Petyuk said, “research has been moving away from a single drug target towards focusing more on the proteins that have a role in cognitive resilience.”
Cognitive resilience is a measure of the brain’s ability to continue to work even with a high Alzheimer’s disease neuropathology that would normally produce the hallmark dementia. This means that, in some people, the brain shows the symptoms of the disease, but it does not impact the person’s ability to function. What makes some brains sensitive, and some resilient is an open question.
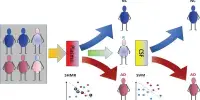
Petyuk recently collaborated with a multi-institutional team in a study that examined a large Alzheimer’s disease cohort of over 1800 people. The researchers drew on previously collected blood samples and brain tissue, along with large-scale data analysis to search for central themes in early identification, prevention, and treatment of the disease.
The research findings published in Science Advances (November 2022), help explain the progression of Alzheimer-related dementia in each patient. Further, the findings outline a multilevel biological classification system that predicts disease severity and future neurological symptoms. “Assessment of a patient’s brain and blood proteins, and other biological molecules, reveal patterns that can then be targeted for tailored intervention,” said Petyuk.
The discovery is particularly timely, as November is Alzheimer’s disease awareness month. In the United States, 5.4 million people aged 65 and older live with Alzheimer’s disease. The numbers grow annually as the population ages.
The right tools, at the right time, in the right place
These types of large-scale studies, exploring proteins and protein-related data are often called proteomics studies.
Proteomics research at PNNL involves, among other things, the ability to analyze very large data sets. Examining, identifying, and discovering proteins can answer specific biological questions about their role in the disease, as well as identifying multiple new drug targets in the fight against Alzheimer’s disease and related dementias.
Leveraging PNNL’s advanced proteomics platform capabilities to answer these big questions to fill in the knowledge gaps, Petyuk has contributed to six published research studies in this year alone. The work validates the power of discovery in the proteomics platform at PNNL, as well as the power in the collaborative efforts of Petyuk’s colleagues from all over the world.
Putting together pieces of the Alzheimer’s puzzle
Some symptoms of the disease are due to the misfolding of proteins. Proteins need to have a specific shape to function correctly, and much like baking a cake, changing the recipe can result in a misshapen product. Alzheimer’s disease can cause protein recipes to change. This research adds to the emerging body of work on proteins involved in cognitive decline that are associated with the disease. These proteins may indicate potential new targets for drug therapies.
Even with such a large body of work, the puzzle still only gets put together one piece at a time, with lots of smaller parts that make sense, but a yet-to-be-discovered larger view. Petyuk, along with team lead Yasser Iturria-Medina at the Montreal Neurological Institute of McGill University, continue work that adds to our understanding of a complex and devastating disease. This promises new discoveries, and new pieces to add to the puzzle of Alzheimer’s disease.