Obesity has rapidly increased in recent decades to affect more than 2 billion people, making it one of the leading causes of poor health worldwide. Many people continue to struggle to lose weight despite decades of research on diet and exercise treatments. Researchers at Baylor College of Medicine and other affiliated institutions believe they have discovered why, and they believe we must shift our focus from obesity treatment to prevention.
Obesity has been shown in studies to have an impact on brain health from childhood to adulthood, affecting everything from executive function skills (the complex ability to initiate, plan, and carry out tasks) to significantly increasing dementia risk. By middle age, the consequences of being overweight are severe. Several studies have found that middle-aged adults with a BMI of 30 or higher, indicating obesity, are more likely to develop dementia than their healthy-weight peers.
Obesity has rapidly increased in recent decades to affect more than 2 billion people, making it one of the leading causes of poor health worldwide. Many people continue to struggle to lose weight despite decades of research on diet and exercise treatments. Researchers at Baylor College of Medicine and other affiliated institutions believe they have discovered why, and they believe we must shift our focus from obesity treatment to prevention.
Our study is the first to compare this epigenetic development in males and females. We were surprised to find extensive sex differences. In fact, in terms of these postnatal epigenetic changes, males and females are more different than they are similar. And, many of the changes occurred earlier in females than in males, indicating that females are precocious in this regard.
Dr. Robert Waterland
The team reports in the journal Science Advances that early brain development molecular mechanisms are likely a major determinant of obesity risk. Previous large human studies have suggested that the genes most strongly associated with obesity are expressed in the developing brain. The current mouse study focused on epigenetic development. Epigenetics is a molecular bookmarking system that determines which genes are used or not used in different cell types.
“Decades of research in humans and animal models have shown that environmental influences during critical periods of development have a major long-term impact on health and disease,” said corresponding author Dr. Robert Waterland, professor of pediatrics-nutrition and a member of the USDA Children’s Nutrition Research Center at Baylor. “Body weight regulation is very sensitive to such ‘developmental programming,’ but exactly how this works remains unknown.”
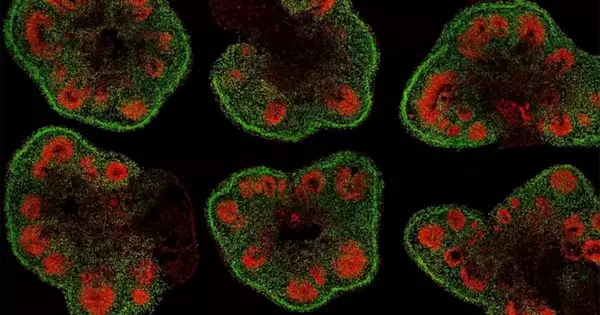
“In this study we focused on a brain region called the arcuate nucleus of the hypothalamus, which is a master regulator of food intake, physical activity, and metabolism,” said first author Dr. Harry MacKay, who was a postdoctoral associate in the Waterland lab while working on the project. “We discovered that the arcuate nucleus undergoes extensive epigenetic maturation during early postnatal life. This period is also exquisitely sensitive to developmental programming of body weight regulation, suggesting that these effects could be a consequence of dysregulated epigenetic maturation.”
The team conducted genome-wide analyses of both DNA methylation – an important epigenetic tag and gene expression, both before and after closure of the postnatal critical window for developmental programming of body weight. “One of our study’s biggest strengths is that we studied the two major classes of brain cells, neurons and glia,” MacKays said. “It turns out that epigenetic maturation is very different between these two cell types.”
“Our study is the first to compare this epigenetic development in males and females,” Waterland said. “We were surprised to find extensive sex differences. In fact, in terms of these postnatal epigenetic changes, males and females are more different than they are similar. And, many of the changes occurred earlier in females than in males, indicating that females are precocious in this regard.”
The human connection
The biggest surprise came when the investigators compared their epigenetic data in mice to human data from large genome-wide association studies that screen for genetic variants associated with obesity. The genomic regions targeted for epigenetic maturation in the mouse arcuate nucleus overlapped strongly with human genomic regions associated with body mass index, an index of obesity.
“These associations suggest that obesity risk in humans is determined in part by epigenetic development in the arcuate nucleus,” MacKay said. “Our results provide new evidence that developmental epigenetics is likely involved in both early environmental and genetic influences on obesity risk. Accordingly, prevention efforts targeting these developmental processes could be the key to stopping the worldwide obesity epidemic.”