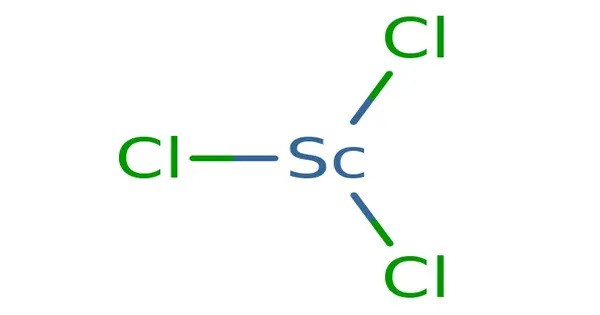
Scandium(III) chloride is the inorganic compound with the formula ScCl3. It is a white, high-melting ionic compound, which is deliquescent and highly water-soluble. This salt is mainly of interest in the research laboratory. Both the anhydrous form and hexahydrate (ScCl3•6H2O) are commercially available.
Scandium(III) chloride is found in some halide lamps, optical fibers, electronic ceramics, and lasers.
Structure
ScCl3 crystallises in the layered BiI3 motif, which features octahedral scandium centres. Monomeric ScCl3 is the predominant species in the vapour phase at 900 K, the dimer Sc2Cl6 accounts for approximately 8%. The electron diffraction spectrum indicates that the monomer is planar and the dimer has two bridging Cl atoms each Sc being 4 coordinate.
Properties
- Chemical formula: ScCl3
- Molar mass: 151.31 g/mol
- Appearance: grayish-white crystals
- Density: 2.39 g/mL, solid
- Melting point: 960 °C (1,760 °F; 1,230 K), 63 °C (hexahydrate)
- Solubility in water: 70.2 g/100 mL
- Solubility in other solvents: soluble in alcohol, acetone, glycerin,
- Insoluble in EtOH
Reduction
Scandium(III) chloride was used by Fischer et al. who first prepared metallic scandium by electrolysis of a eutectic melt of scandium(III) chloride and other salts at 700-800 °C.
ScCl3 reacts with scandium metal to give a number of chlorides where scandium has an oxidation state <+3, ScCl, Sc7Cl10, Sc2Cl3, Sc5Cl8 and Sc7Cl12. For example, reduction of ScCl3 with scandium metal in the presence of caesium chloride gives the compound CsScCl3 which contain linear chains of composition ScIICl3−, containing ScIICl6 octahedra sharing faces.
Preparation
Scandium chloride can be prepared by reacting scandium metal or scandium oxide (Sc₂O₃) with hydrochloric acid (HCl): Sc2O3+6HCl→2ScCl3+3H2O
Alternatively, it can be obtained from scandium minerals by chemical extraction methods.
Occurrences
Scandium is a rare, lightweight metal typically found in trace amounts in minerals like thortveitite, and sometimes in bauxite and uranium ores. Scandium chloride is obtained as a product of processing these ores. It is also produced in small quantities during the extraction of other metals.
Commercial Production
Scandium chloride is not commonly found in large quantities in nature. Instead, scandium itself is extracted from ores and refined to produce scandium chloride. This compound is often used in specialized applications, including as a catalyst, in metal alloys, and in the production of high-performance materials for aerospace and electronics industries.
Uses
- Catalysis: Scandium chloride is used as a catalyst in various chemical reactions, including in the production of aluminum alloys and other specialty chemicals.
- Material Science: Scandium(III) chloride is a precursor for other scandium compounds, such as scandium oxide, which has applications in high-performance alloys and certain electronics.
- Solid-State Lasers: Scandium chloride plays a role in some laser technology, specifically in solid-state lasers, due to its optical properties.
Reactivity
- Scandium chloride is quite stable under normal conditions but is highly reactive when exposed to strong bases.
- It reacts with water to form hydrochloric acid and scandium hydroxide: ScCl3+3H2O→Sc(OH)3+3HCl
Safety
Scandium chloride is generally considered to have low toxicity, but like many chemicals, it should be handled with care, especially in its powdered or concentrated form. It is recommended to wear appropriate protective equipment to avoid irritation and inhalation.