Researchers created a label-free, non-invasive Raman spectroscopy method that can acquire microscopic images of biological samples and identify a wide range of biomolecules with unprecedented speed and sensitivity.
“Our research could lead to a non-invasive, label-free, and user-friendly device for clinical use,” said Dario Polli of the Politecnico di Milano in Italy. “By allowing visualization of the chemical constituents of human tissues and cells, this innovative microscope, combined with deep learning-based algorithms, could eventually make cancer diagnosis easier and faster.”
The researchers describe their new technique, which is based on coherent anti-stokes Raman scattering (CARS) microscopy, in the Optica Publishing Group journal Optics Express. CARS microscopy uses the interaction of ultrashort laser pulses and biological samples to generate images based on the vibrational signatures of molecules.
The new method allows access to the difficult-to-detect fingerprint region of the vibrational spectrum, which ranges from 400 to 1800 cm-1. Although many individual compounds can be identified in this region using their vibrational fingerprints, it produces weak signals that are difficult to detect.
“Commonly used techniques in biomedical sciences often require staining, which is not only cumbersome but can also introduce structural and chemical alterations that can lead to artifacts, or errors, in imaging and data processing,” said Polli. “Because our system can distinguish between many different chemical species in biological tissues without labels, it could be useful for live cell imaging and analyzing tissue biopsies.”
Our CARS microscope enables label-free imaging with chemical specificity at faster speeds, making Raman imaging of living cells more feasible. For example, our system could be used to analyze the interactions of cancer cells with immune cells or to characterize how chemotherapy affects cells.
Dario Polli
Lower repetition rate, faster imaging
This new research is part of the European Commission-funded CRIMSON project, which aims to develop a turnkey imaging device that uses vibrational spectroscopy for fast cell and tissue classification. The project’s goal is to revolutionize research into the cellular origins of diseases in order to enable new approaches that could advance personalized therapy.
The researchers took an important step toward this goal by developing a CARS microscope based on a commercial laser that generates ultrashort pulses with durations of approximately 270 femtoseconds in the near-infrared wavelength range. The microscopy system was designed to use laser pulses with a repetition rate of 2 MHz, which is significantly lower than the 40 or 80 MHz used by most other CARS systems.
This lower repetition rate reduces photothermal damage to the sample because it creates a delay of 0.5 microseconds between two consecutive pulses. It also produces a higher pulse energy and peak intensity at the focal point, which generates a stronger CARS signal and allows a faster acquisition speed.
“The most important advantage of the lower repetition rate is that it allowed us to generate broadband, red-shifted Stokes pulses that cover the whole fingerprint vibrational region by using white-light supercontinuum generation in a bulk crystal,” said Federico Vernuccio, doctoral student at Politecnico di Milano and first author of the study. “Compared to other methods, this approach is technically simpler, more compact and robust.”
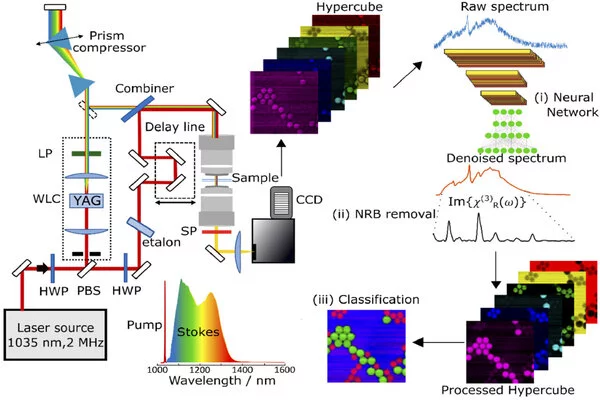
Using a red-shifted spectral region in comparison to standard setups allows for higher laser intensities to be used before photodamage occurs. In addition, the researchers created new algorithms that combine traditional numerical computational approaches with artificial intelligence. These algorithms extract additional information from the collected data and convert it into images that allow different chemical species to be distinguished.
“Thanks to our improvements, the CARS system now delivers high-quality images at cutting-edge acquisition speeds,” Vernuccio said. “Our system has a pixel dwell time of less than 1 millisecond without compromising sample integrity. The spectrometer refresh rate limits this speed.”
High-speed sensitivity
To test their system, the researchers used reference samples to compare spectra retrieved with the new microscope with ones acquired using a state-of-the-art, although slower, vibrational spectroscopy technique. The two methods showed excellent agreement, demonstrating that the new system could deliver spectra at very high speeds with good spectral resolution and chemical specificity.
The researchers then determined the detection limit of their system by acquiring CARS spectra of a variety of dimethyl sulfoxide solutions at various concentrations. The system measured chemical concentration with an unprecedented sensitivity of 14.1 mmol/liter, roughly double the sensitivity of other CARS systems working in the fingerprint region.
They also demonstrated the system’s ability to distinguish and spatially localize various transparent micron-sized plastic beads based on their vibrational signature, as well as measurements from biological tissues to demonstrate that the technique works on biological samples without causing damage.
“Our CARS microscope enables label-free imaging with chemical specificity at faster speeds, making Raman imaging of living cells more feasible,” Polli explained. “For example, our system could be used to analyze the interactions of cancer cells with immune cells or to characterize how chemotherapy affects cells.”
The researchers are now working to improve their system by generating Stokes pulses with a wider wavelength range using white-light supercontinuum generation. This would increase both the imaging speed and the number of detectable chemical analytes. They are also developing user-friendly software, compact optical sources, and designs for a commercial prototype and detection system in order to commercialize their work.