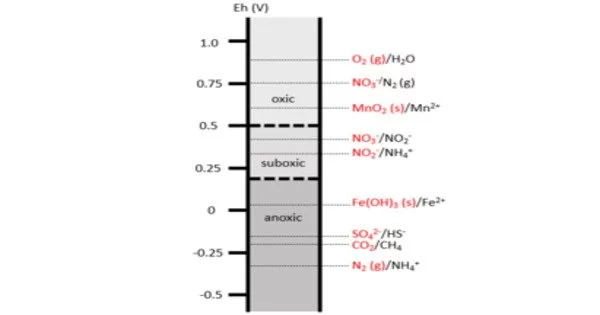
A redox gradient is a variation in the redox potential of a system along a spatial or temporal axis. Redox potential is a measure of the tendency of a chemical species to gain or lose electrons, and it is commonly expressed in volts (V) or millivolts (mV).
In environmental science, redox gradients are often observed in aquatic systems where oxygen concentration varies with depth. In such systems, there is a gradual decrease in the concentration of oxygen as depth increases, and an increase in the concentration of reduced compounds such as sulfides or iron ions. The redox gradient in aquatic systems is important for determining the distribution of organisms in the water column and the biogeochemical cycling of nutrients.
In natural systems, such as soils, sediments, and water bodies, redox gradients can be created by the presence of different electron acceptors and donors, such as oxygen, nitrate, sulfate, iron, and manganese. Microbial activity plays a crucial role in mediating these redox reactions, as microorganisms use these electron acceptors and donors as sources of energy for metabolic processes.
A redox gradient is a series of reduction-oxidation (redox) reactions sorted by redox potential. The redox ladder depicts the order in which redox reactions occur based on the free energy gained from redox pairs. These redox gradients form both spatially and temporally as a result of differences in microbial processes, chemical composition of the environment, and oxidative potential. Redox gradients are common in coastal marshes, lakes, contaminant plumes, and soils.
Redox gradients can have important implications for biogeochemical cycling, nutrient availability, and pollutant remediation in natural systems. For example, the presence of a redox gradient can create zones of different microbial activity and biogeochemical transformations, leading to the formation of distinct ecological niches and biogeochemical hotspots. In addition, redox gradients can affect the mobility and speciation of contaminants, such as heavy metals and organic pollutants, by altering their oxidation state and solubility.
Redox gradients are also important in bioremediation, where microorganisms use redox reactions to transform and degrade pollutants. For example, some microorganisms can use the oxygen gradient in soil to degrade organic pollutants through aerobic respiration, while others can use the redox gradient created by the presence of nitrate or sulfate ions to carry out anaerobic biodegradation.
Overall, redox gradients are an important concept in understanding the distribution and behavior of chemicals and organisms in natural and engineered systems. The Earth has a global redox gradient, with oxidizing conditions at the surface and increasingly reducing conditions beneath the surface. At the macro level, redox gradients are well understood; however, characterization of redox reactions in heterogeneous environments at the micro-scale necessitates additional research and more sophisticated measurement techniques.