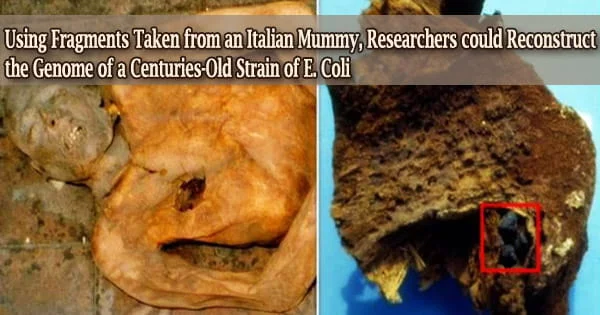
Definition –
Realgar, α-As4S4, also known as “ruby sulphur” or “ruby of arsenic”, an important ore of arsenic, a red or orange mineral containing both arsenic and sulfur. It is a soft, sectile mineral occurring in monoclinic crystals, or in granular, compact, or powdery form, often in association with the related mineral, orpiment (As2S3). It is orange-red in color, melts at 320 °C, and burns with a bluish flame releasing fumes of arsenic and sulfur.
Realgar is soft with a Mohs hardness of 1.5 to 2 and has a specific gravity of 3.5. Its streak is orange colored. It is trimorphous with alacranite and pararealgar. Its name comes from the Arabic rahj al-ġār (رهج الغار, “powder of the mine”), via Catalan and Medieval Latin, and its earliest record in English is in the 1390s.
Typically it is a minor constituent of ore veins in association with orpiment (into which it disintegrates on long exposure to light). Realgar has been used by the Chinese for carvings, but these also deteriorate under light. It forms prismatic crystals of monoclinic symmetry.
Due to the instability of Realgar, specimens should be stored enclosed and covered to prevent their exposure to light. Occasional exposure to look at a specimen will not cause damage; only prolonged or repeated exposure will cause alteration. Several important museums have had Realgar on display consistently exposed to light, and these specimens can be seen altered and crumbled.

Occurrence and Properties of Realgar –
Realgar most commonly occurs as a low-temperature hydrothermal vein mineral associated with other arsenic and antimony minerals. It also occurs as volcanic sublimations and in hot spring deposits. It occurs in association with orpiment, arsenolite, calcite, and barite. It is found with lead, silver and gold ores in Hungary, Bohemia, and Saxony. In the US it occurs notably in Mercur, Utah; Manhattan, Nevada and in the geyser deposits of Yellowstone National Park.
Realgar crystals are prismatic and stubby, and maybe in irregular groups. Crystals are usually heavily striated lengthwise and maybe well-terminated or rectangular in shape. Also drusy, encrusting, botryoidal, grainy, massive, and in earthy masses.
Realgar is commonly held that after a long period of exposure to light realgar changes form to a yellow powder known as pararealgar (β-As4S4). It was once thought that this powder was the yellow sulfide orpiment, but has been recently shown to be a distinct chemical compound.
Some of the best Realgar crystals come from the Rumanian Transylvania at Baia Sprie (Felsöbánya), as well as Cavnic (Kapnik), both in Maramures Co. Outstanding transparent gemmy crystals are well known from the Jiepaiyu (Shimen) Mine, Hunan Province, China. Bright red though small Realgar crystals in contrasting white marble came from the Lengenbach Quarry, Wallis, Switzerland. Realgar occurs with Colmanite in the borate deposit of Bigadiç, Marmara Region, Turkey; and it occurs in dark red crystals in the Palomo Mine, Huancavelica, Peru.
The chemical formula of realgar is often written as As4S4 instead of the simpler AsS. This is done because As4S4 represents a structural unit of the mineral. A compound of As+3 and S-2 would be out of electrical balance. In realgar, three of the arsenics are joined in a chain by covalent bonds. This gives the arsenics an effective electrical charge of +8. That combines with four S-2 ions to produce an electrically neutral molecule. This is why realgar’s chemical composition is often presented as As4S4 instead of AsS.
Uses of Realgar –
Realgar was used by firework manufacturers to create the color white in fireworks prior to the availability of powdered metals such as aluminum, magnesium and titanium; because ‘Realgar’ is an important ore of arsenic. It is still used in combination with potassium chlorate to make a contact explosive known as “red explosive” for some types of torpedoes and other novelty exploding fireworks branded as ‘cracker balls’, as well in the cores of some types of crackling stars.
However, Realgar is not used as a gemstone because it is very soft, with a Mohs hardness of just 1-1/2 to 2. It is easily ground into a fine, bright red powder. Those properties caused it to become a favorite pigment in many parts of the ancient world. It was traded over great distances to make paints, inks, and dyes until people realized that it was toxic.
Realgar is toxic. It is may sometimes be used to kill weeds, insects, and rodents, even though more effective arsenic-based agents are available. It was commonly applied in leather manufacturing to remove the hair from animal pelts. Because realgar is a known carcinogen, and an arsenic poison, and because competitive substitutes are available, it is rarely used today for this purpose.
Historical uses of Realgar: The ancient Greeks, who called it sandaracha, understood that it was poisonous. It was used to poison rats in medieval Spain and in 16th-century England. From this, realgar has also historically been known in English as sandarac.
Realgar was also used by Ancient Greek apothecaries to make a medicine known as “bull’s blood.” The Greek physician Nicander described death by bull’s blood, which matches the known effects of arsenic poisoning. Bull’s blood is the poison that is said to have been used by Themistocles and Midas for suicide. Realgar was, along with orpiment, a significant item of trade in the ancient Roman Empire and was used as a red paint pigment. Early occurrences of realgar as a red painting pigment are known for works of art from China, India, Central Asia, and Egypt. It was used in European fine-art painting during the Renaissance era, a use which died out by the 18th century.
However, Realgar was also used in leather processing to remove the hair from hides. Today it is rarely used for any of these purposes because more effective and less toxic substitutes have been developed. However, some use of realgar in ritualistic cosmetics and “medicines” continues in a few parts of the world. Today the main use of realgar is as an ore of arsenic metal.
Information Sources: