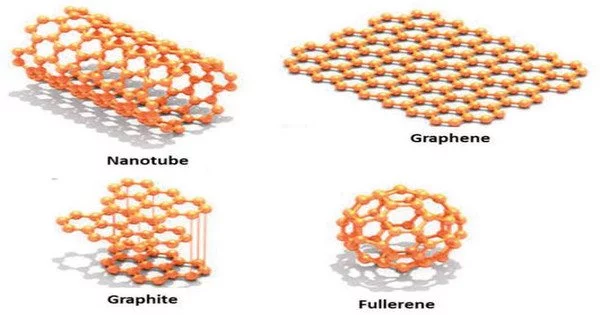
Two-dimensional (2D) nanoparticles, also known as nanosheets or nanofilms, have recently gained a lot of attention due to their unique properties and potential applications in various fields. These nanoparticles have a thickness of only a few atomic layers and a large surface area, which makes them ideal for a wide range of applications.
A research team discovered how to produce catalysts and many other nanoplatelets in an environmentally friendly manner using readily available materials in sufficient quantities.
Hydrogen is regarded as a more environmentally friendly alternative to traditional fossil fuels. Until now, expensive and rare materials such as platinum were required for catalytic production, such as electrolytic water splitting. More readily available catalysts may make large-scale production possible in the future.
The research teams of Helmut Cölfen (Physical Chemistry) and Peter Nielaba (Statistical and Computational Physics) at the University of Konstanz have developed a general method to produce two-dimensional nanoparticles from readily accessible materials, together with researchers from the Ocean University of China, Qingdao (China) and the Fritz Haber Institute of the Max Planck Society, Berlin (Germany). Two-dimensional nanoparticles have a high catalytic potential, which is why this synthetic route is suitable for producing particularly active catalysts.
The research team has discovered how the concentration of molecular building blocks in the solution can be used to manipulate this process: If the concentration of building blocks is increased, the principle of “what grows fast also consumes more material” comes into play: The distance between the fast-growing and the slower growing crystal axes increases, resulting in two-dimensional particles.
The corresponding synthesis takes place in a simple aqueous solution. There are no toxic additives or particularly high temperatures that are inefficient in terms of energy. The process is simply controlled by varying the concentration of the components and regulating the temperature. Using this method, the researchers were able to shape more than 30 different compounds into two-dimensional forms, which was published for the first time in the scientific journal Nature Synthesis.
The advantage of two-dimensional nanoparticles
Two-dimensional (2D) nanoparticles have a disproportionately large number of surface atoms, which have distinct properties from atoms within the particle. Because the surface lacks the immediate neighboring atoms to which bonds are formed inside the particle, the bonds of the surface atoms are non-saturated. This causes surface tension or interfacial tension. Because this non-saturated state consumes a lot of energy for the entire system, nanoparticles try to cluster together to saturate the bonds and reduce the surface area.
However, if the surface bonds remain unsaturated, the chemical reactivity increases. The number of unsaturated bonds in two-dimensional nanoparticles is especially high because they have unsaturated bonds not only at the top and bottom but also at the sides and edges. This makes them particularly appealing for catalysis, which is important in chemistry. However, due to the unfavorable energy state at the surface, the required nanocrystals are difficult to fabricate.
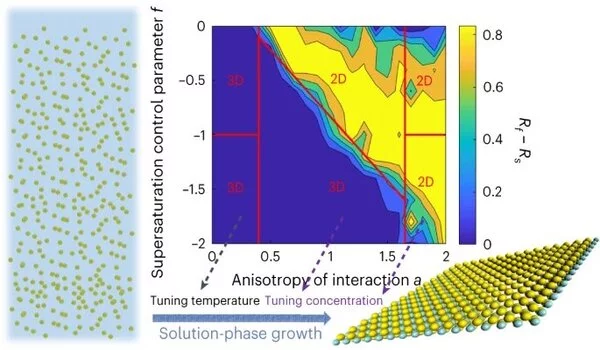
Anisotropic two-dimensional nanoparticles have properties that depend on the orientation of their building blocks. The crystal lattice of the particles determines the direction of their growth. The particles grow two-dimensionally if the nanoparticles have a layered crystal lattice, as in clay. However, catalytically active materials rarely take on a two-dimensional shape on their own. Two-dimensional nanoparticles can be easily synthesized if the crystal lattice dictates that the crystal grows rapidly along two crystal axes. The solution then only requires a few molecular building blocks to grow the nanoparticles two-dimensionally. If the crystals grow in other directions at the same or slightly slower rate, they will take on a three-dimensional shape.
How nanoparticles grow two-dimensionally
The research team has discovered how the concentration of molecular building blocks in the solution can be used to manipulate this process: If the concentration of building blocks is increased, the principle of “what grows fast also consumes more material” comes into play: The distance between the fast-growing and the slower growing crystal axes increases, resulting in two-dimensional particles.
If the growth rate along different relevant crystal axes is approximately the same, the method of increasing building block concentration does not work. In this case, the researchers employ a different parameter. The rate of growth of crystal surfaces is proportional to temperature. The difference in growth rates between the slow and fast-growing crystal faces will increase if the temperature of the solution is changed by even a few degrees. As a result, the nanoparticles become two-dimensional.
Method works for over 30 elements of the periodic table
This general procedure is applicable to a wide range of materials. The German-Chinese research team was able to identify metals in many groups, more than 30 in total, that take the two-dimensional form as oxides or hydroxides, but also acids, sulfides, oxychlorides, and phosphates, in the periodic table. The following are the benefits of this new general approach: Most materials are created at room temperature in water, without the use of toxic solvents or high temperatures.
Furthermore, catalytic material yield is highly scalable. The researchers are working on a multigram scale in the lab. To produce catalysts in large quantities using readily available materials, all that is required is a sealed vessel rather than specialized equipment such as pressure vessels.